All Photos(1)
About This Item
Linear Formula:
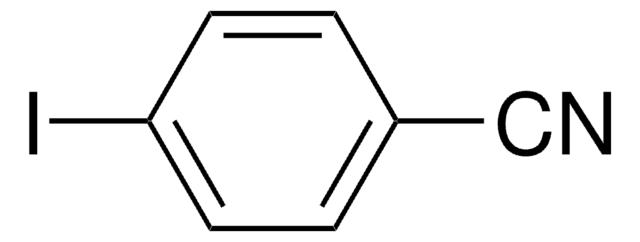
NCC6H4C6H4CN
CAS Number:
Molecular Weight:
204.23
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
236-240 °C (lit.)
SMILES string
N#Cc1ccc(cc1)-c2ccc(cc2)C#N
InChI
1S/C14H8N2/c15-9-11-1-5-13(6-2-11)14-7-3-12(10-16)4-8-14/h1-8H
InChI key
KAXYYLCSSXFXKR-UHFFFAOYSA-N
General description
4,4′-Biphenyldicarbonitrile (BPCN), also known as 4,4′-dicyanobiphenyl, is a para-disubstituted biphenyl. It can be prepared from benzidine dihydrochloride.3 BPCN belongs to the monoclinic crystal system and P21 space group.
Application
4,4′-Biphenyldicarbonitrile (BPCN, 4,4′-dicyanobiphenyl) may be used in the preparation of:
- [Ag(BPCN)2]PF6 (PF6=Hexafluorophosphate)
- 4,4′-diphenacetylbiphenyl
- triazine-framework-based porous membranes (TFMs)
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Crystallization of 4,4?-biphenyldicarbonitrile with silver (I) salts: a change in topology concomitant with a change in counterion leading to a ninefold diamondoid network.
Hirsch KA, et al.
Journal of the Chemical Society. Chemical Communications, 21, 2199-2200 (1995)
Bistetracyclones? and ?Bishexaphenylbenzenes.
Ogliaruso MA, et al.
The Journal of Organic Chemistry, 28(10), 2725-2728 (1963)
A superacid-catalyzed synthesis of porous membranes based on triazine frameworks for CO2 separation.
Zhu X, et al.
Journal of the American Chemical Society, 134(25), 10478-10484 (2012)
4, 4?-Dicyanobiphenyl.
Britton D and Young Jr VG.
Acta Crystallographica Section E, Structure Reports Online, 59(11), o1849-o1851 (2003)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service








![(S,S)-(+)-2,6-Bis[2-(hydroxydiphenylmethyl)-1-pyrrolidinyl-methyl]-4-methylphenol 95%](/deepweb/assets/sigmaaldrich/product/structures/126/939/bff1a61c-8335-434f-8da4-9b1929aef17f/640/bff1a61c-8335-434f-8da4-9b1929aef17f.png)