All Photos(1)
About This Item
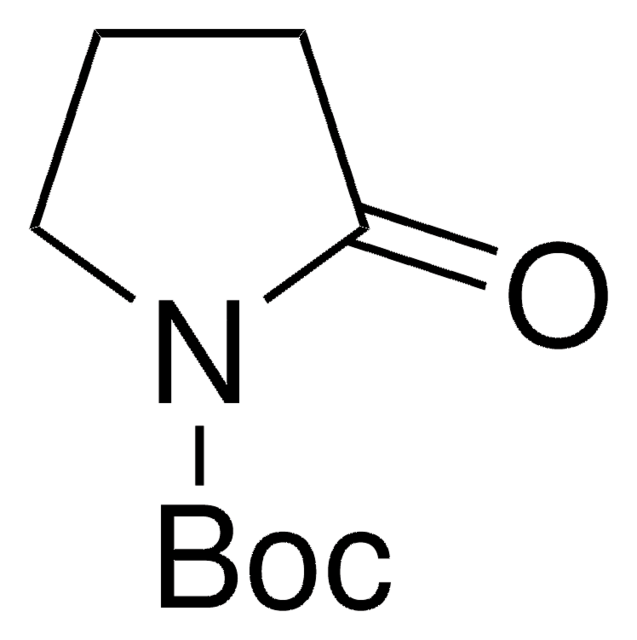
Empirical Formula (Hill Notation):
C10H16N2O2
CAS Number:
Molecular Weight:
196.25
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
optical activity
[α]20/D −105.5°, c = 1 in chloroform
mp
33-36 °C (lit.)
SMILES string
CC(C)(C)OC(=O)N1CCC[C@H]1C#N
InChI
1S/C10H16N2O2/c1-10(2,3)14-9(13)12-6-4-5-8(12)7-11/h8H,4-6H2,1-3H3/t8-/m0/s1
InChI key
MDMSZBHMBCNYNO-QMMMGPOBSA-N
Application
Used in synthesis of prolyl oligopeptidase inhibitors.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Janice Lawandi et al.
Journal of medicinal chemistry, 52(21), 6672-6684 (2009-11-06)
Prolyl oligopeptidases cleave peptides on the carboxy side of internal proline residues and their inhibition has potential in the treatment of human brain disorders. Using our docking program fitted, we have designed a series of constrained covalent inhibitors, built from
Erik A A Wallén et al.
Journal of medicinal chemistry, 46(21), 4543-4551 (2003-10-03)
Isophthalic acid bis(l-prolyl-pyrrolidine) amide is a very potent prolyl oligopeptidase inhibitor, but it has a log P value of -0.2, which is very low for a compound targeted to the brain. Therefore, these types of compounds were further modified to
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service