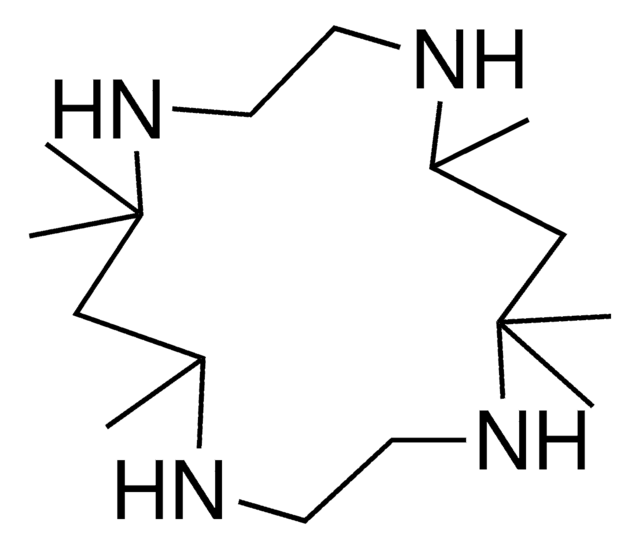
526169
1,8-Dimethyl-1,4,8,11-tetraazacyclotetradecane
Synonym(s):
1,8-Dimethylcyclam
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H28N4
CAS Number:
Molecular Weight:
228.38
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
refractive index
n20/D 1.4940 (lit.)
bp
98-99 °C (lit.)
density
0.944 g/mL at 25 °C (lit.)
SMILES string
CN1CCCNCCN(C)CCCNCC1
InChI
1S/C12H28N4/c1-15-9-3-5-14-8-12-16(2)10-4-6-13-7-11-15/h13-14H,3-12H2,1-2H3
InChI key
BNLDMZVBFXARKJ-UHFFFAOYSA-N
Related Categories
General description
1,8-Dimethyl-1,4,8,11-tetraazacyclotetradecane is a heterocyclic building block.
Application
1,8-Dimethyl-1,4,8,11-tetraazacyclotetradecane may be used as a precursor for the synthesis of `trans′-difunctionalized derivatives. It may also be used in the synthesis of 4,11-bis(N-pyren-1-yl-acetamide)-1,8-dimethyl-1,4,8,11-tetraazacyclotetradecane.
Reactant for:
- Ferromagnetic coupling of copper(II) and nickel(II) complexes
- Preparation of cyclam bridged dinuclear platinum(II) complex as an antitumor agent
- Preparation of dimethylcyclam based fluoroionophore having Hg2+- and Cd2+-selective signaling behaviors
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
195.1 °F - closed cup
Flash Point(C)
90.6 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Copper (II) and Nickel (II) Complexes oftrans'-Difunctionalized Tetraaza Macrocycles.
Comparone A and Kaden TA.
Helvetica Chimica Acta, 81(10), 1765-1772 (1998)
So-Youn Moon et al.
The Journal of organic chemistry, 70(6), 2394-2397 (2005-03-12)
[reaction: see text] A new fluoroionophore has been synthesized by appending two signaling pyrenylacetamide subunits on the binding motif of 1,8-dimethylcyclam. The designed compound exhibited highly selective and sensitive fluoroionophoric behavior toward Hg(2+) ions of excimer emission in aqueous dioxane
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service