471860
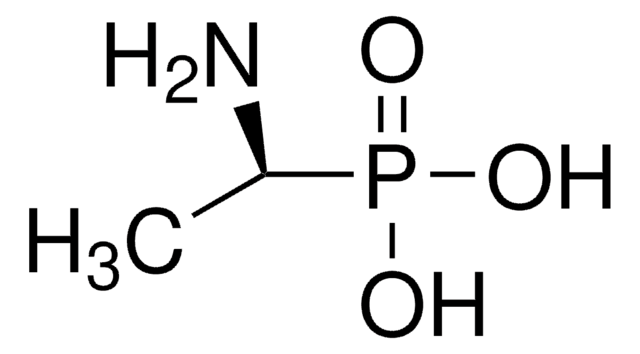
(1R)-(+)-(1-Amino-2-methylpropyl)phosphonic acid
98%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2CHCH(NH2)P(O)(OH)2
CAS Number:
Molecular Weight:
153.12
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
optical activity
[α]20/D +1.0°, c = 1 in 1 M NaOH
mp
272-277 °C (lit.)
SMILES string
CC(C)[C@H](N)P(O)(O)=O
InChI
1S/C4H12NO3P/c1-3(2)4(5)9(6,7)8/h3-4H,5H2,1-2H3,(H2,6,7,8)/t4-/m1/s1
InChI key
DGSLPJDIFKVSIB-SCSAIBSYSA-N
Related Categories
General description
The enantiodiscrimination of (1R)-(+)-(1-amino-2-methylpropyl)phosphonic acid from its enantiomer in the gas-phase can be done using ESI-MS-CID technique. [electrospray ionization-mass spectrometric detection-collision induced dissociation]
Application
Serves as an attractive substitute for amino carboxylic acids in biological systems. Exhibits interesting and useful properties as peptide analog, antiviral agent, hapten for the generation of catalytic antibodies, enzyme inhibitors, potent antibiotics, herbicides, and pesticides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Enantiodiscrimination of chiral a-aminophosphonic acids by mass spectrometry.
Paladini A, et al.
Chirality, 13(10), 707-711 (2001)
Nour Z Atwany et al.
Biology, 9(8) (2020-08-13)
Regulatory T cells (Tregs) are key players in the regulation of inflammatory responses. In this study, two natural molecules, namely, sparteine sulfate (SS) and harpagoside (Harp), were investigated for their ability to induce Tregs in human peripheral blood mononuclear cells
Camp, N.P. et al.
Bioorganic & Medicinal Chemistry Letters, 2, 1047-1047 (1992)
M C Allen et al.
Journal of medicinal chemistry, 32(7), 1652-1661 (1989-07-01)
The synthesis of five amino phosphorus derivatives, 1a-e, is described. The derivatives were incorporated into a series (18) of analogues of the 5-14 portion of angiotensinogen, in most cases at the scissile Leu-Val bond. The resultant compounds were tested in
J Bird et al.
Journal of medicinal chemistry, 37(1), 158-169 (1994-01-07)
The synthesis of a series of N-phosphonalkyl dipeptides 6 is described. Syntheses were devised that allowed the preparation of single diastereoisomers and the assignment of stereochemistry. The compounds were evaluated in vitro for their ability to inhibit the degradation of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service