176974
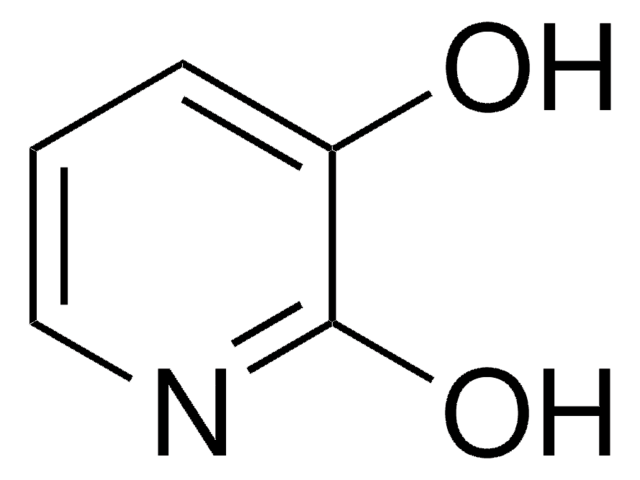
2,4-Dihydroxypyridine
97%
Synonym(s):
2,4-Pyridinediol, 3-Deazauracil, 4-Hydroxy-2-pyridone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H5NO2
CAS Number:
Molecular Weight:
111.10
Beilstein:
108533
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
Recommended Products
Assay
97%
form
solid
mp
272-276 °C (lit.)
SMILES string
Oc1ccnc(O)c1
InChI
1S/C5H5NO2/c7-4-1-2-6-5(8)3-4/h1-3H,(H2,6,7,8)
InChI key
ZEZJPIDPVXJEME-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2,4-Dihydroxypyridine (3-deazauracil) is a potent inhibitor of dihydrouracil dehydrogenase.
Application
2,4-Dihydroxypyridine (3-deazauracil) was used in the synthesis of diazaphenoxathiin skeleton.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
F N Naguib et al.
Biochemical pharmacology, 38(9), 1471-1480 (1989-05-01)
One hundred and five nucleobase analogues were screened as inhibitors of dihydrouracil dehydrogenase (DHUDase, EC 1.3.1.2) from mouse liver. 5-Benzyloxybenzyluracil, 1-deazauracil (2,6-pyridinediol), 3-deazauracil (2,4-pyridinediol), 5-benzyluracil, 5-nitrobarbituric acid and 5,6-dioxyuracil (alloxan) were identified as potent inhibitors of this activity, with apparent
M T Cocco et al.
European journal of medicinal chemistry, 35(5), 545-552 (2000-07-12)
4-hydroxy-2-pyridone derivatives 2 were prepared by reaction of 3-amino-3-dialkylaminopropenoates with bis(2,4, 6-trichlorophenyl)malonate. These compounds were further reacted with a set of aldehydes to give bis(pyridyl)methanes 3 and 4. The newly synthesized compounds 2, 3 and 4 were evaluated in vitro
F P LaCreta et al.
Cancer research, 49(10), 2567-2573 (1989-05-15)
The breakdown of 5-fluoro-2'-deoxyuridine (FdUrd) to 5-fluorouracil (FUra) is catalyzed by the pyrimidine nucleoside phosphorylases, uridine phosphorylase and thymidine phosphorylase. The effects of nucleoside phosphorylase inhibitors on FdUrd and FUra elimination by the isolated perfused rat liver were investigated. The
M Kaneko et al.
Nucleic acids symposium series, (12)(12), 13-16 (1983-01-01)
New method for a synthesis of diazaphenoxathiin skeleton from 3-deazauracil derivatives is reported. It became possible to convert 3-deazauridine to 3-deazacytidine via an excellent intermediate "diazaphenoxathiin sulfoxide derivative".
J Molgó et al.
Journal de pharmacologie, 16 Suppl 2, 109-144 (1985-01-01)
In this review the effects of aminopyridines and chemically related compounds are documented in an attempt to analyse the mechanism underlying their presynaptic actions at the vertebrate neuromuscular junction. Aminopyridines and related compounds are of particular interest because they greatly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service