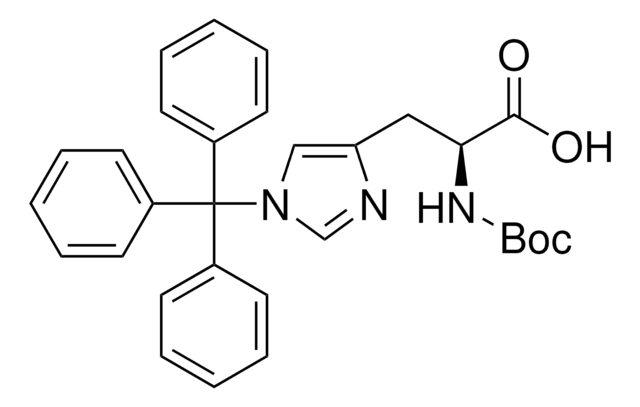
15145
(−)-Bis[(S)-1-phenylethyl]amine hydrochloride
≥98.0% (AT)
Synonym(s):
(−)-Bis[(S)-α-methylbenzyl]amine hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C16H19N · HCl
CAS Number:
Molecular Weight:
261.79
Beilstein:
4723969
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (AT)
form
solid
optical activity
[α]20/D −73±2°, c = 3% in ethanol
mp
~260 °C
SMILES string
Cl.C[C@H](N[C@@H](C)c1ccccc1)c2ccccc2
InChI
1S/C16H19N.ClH/c1-13(15-9-5-3-6-10-15)17-14(2)16-11-7-4-8-12-16;/h3-14,17H,1-2H3;1H/t13-,14-;/m0./s1
InChI key
ZBQCLJZOKDRAOW-IODNYQNNSA-N
Application
(−)-Bis[(S)-1-phenylethyl]amine hydrochloride can be used:
- To prepare phosphoramidite (Feringa) ligand named (R)-2,2′-binaphthoyl-(S,S)-di-(1-phenylethyl)aminoylphosphine.
- As a chiral amphiphilic cation to encapsulate polyoxometalates, which act as supramolecular assemblies employed in the asymmetric oxidation of sulfides.
- As a chiral shift agent in the determination of enantiomeric purity of tris(tetrachlorobenzenediolato) phosphate(V) anion using 31P NMR.
Other Notes
The corresponding chiral lithium amide is used as base for the enantioselective deprotonation of ketones.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Large-scale synthesis and resolution of trisphat [tris (tetrachlorobenzenediolato) phosphate (v)] anion
Favarger F, et al.
The Journal of Organic Chemistry, 69(24), 8521-8524 (2004)
Supramolecular assembly of chiral polyoxometalate complexes for asymmetric catalytic oxidation of thioethers
Wang Y, et al.
Journal of Materials Chemistry, 22(18), 9181-9188 (2012)
P.J. Cox et al.
Tetrahedron Asymmetry, 2, 1-1 (1991)
M. Majewski et al.
The Journal of Organic Chemistry, 57, 3599-3599 (1992)
(R)-2, 2′-Binaphthoyl-(S, S)-DI (1-phenylethyl) aminophosphine. Scalable protocols for the syntheses of phosphoramidite (feringa) ligands
Smith CR, et al.
Organic Syntheses, 85, 238-238 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![(+)-Bis[(R)-1-phenylethyl]amine 99%](/deepweb/assets/sigmaaldrich/product/structures/188/828/177cd49c-056f-47d3-976c-c8cdcd5f62c5/640/177cd49c-056f-47d3-976c-c8cdcd5f62c5.png)
![(−)-Bis[(S)-1-phenylethyl]amine 99%](/deepweb/assets/sigmaaldrich/product/structures/336/455/d6f04f0e-9bcc-4d67-a94d-d153e39209e1/640/d6f04f0e-9bcc-4d67-a94d-d153e39209e1.png)