L2037
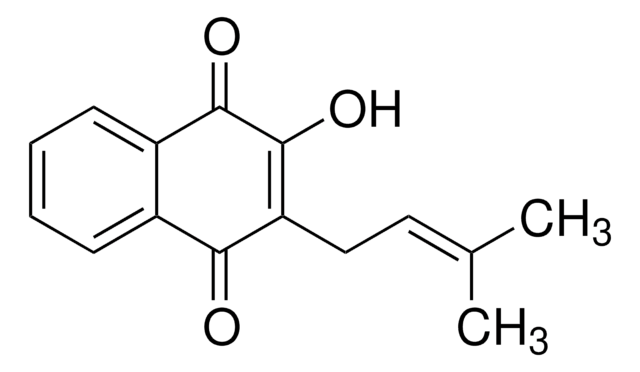
β-Lapachone
≥98% (TLC)
Synonym(s):
ARQ 501, NSC 26326, NSC 629749, SL 11001
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C15H14O3
CAS Number:
Molecular Weight:
242.27
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
biological source
synthetic (organic)
Assay
≥98% (TLC)
form
powder
SMILES string
CC1(C)CCC2=C(O1)c3ccccc3C(=O)C2=O
InChI
1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3
InChI key
QZPQTZZNNJUOLS-UHFFFAOYSA-N
Application
β-Lapachone has been used:
- as an anticancer compound in catalase-inhibitable luminol/hydrogen peroxide (HRP)-dependent chemiluminometric assay in Lewis lung carcinoma (LLC) cells and isolated mitochondria
- as a naphthoquinone to study its effects on the growth and differentiation of mice granulocyte and macrophage progenitor cells
- as a substrate to study the enzyme activity of human recombinant NAD(P)H dehydrogenase 1 (NQO1) protein
Biochem/physiol Actions
β-Lapachone acts as a DNA topoisomerase type I inhibitor. It exhibits anti-fungal, anti-bacterial, trypanocidal, and antiviral properties. β-Lapachone also inhibits nitric oxide (NO) and inducible NO synthase (iNOS) in alveolar macrophages.
β-Lapachone is a naturally occurring quinone obtained from the bark of the lapacho tree (Tabebuia avellanedae) with cancer chemopreventive properties. Induces apoptosis in HL-60 and human prostate cancer cells.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cleverson Neves-Pinto et al.
Journal of medicinal chemistry, 45(10), 2112-2115 (2002-05-03)
An intensive effort has been directed toward finding alternative drugs for treatment of Chagas' disease, caused by Trypanosoma cruzi, and prophylaxis of blood in endemic areas. Our research comprises the synthesis and trypanocidal screening of derivatives from naphthoquinones. Herein a
Byung Tae Choi et al.
Anti-cancer drugs, 14(10), 845-850 (2003-11-05)
beta-Lapachone is a naturally occurring quinone obtained from the bark of the lapacho tree (Tabebuia avellanedae) with cancer chemopreventive properties. The objective of the present study was to investigate the effect of beta-lapachone on the cell growth and apoptosis in
Mary L S Queiroz et al.
Journal of ethnopharmacology, 117(2), 228-235 (2008-03-18)
The effects of Tabebuia avellanedae (TACE), traditionally prescribed in the treatment of cancer, and the naphtoquinone beta-lapachone (beta-lap) on the growth and differentiation of granulocyte and macrophage progenitor cells (CFU-GM) were studied in Ehrlich ascites tumour-bearing mice. Myelosuppression concomitant with
Carla Ríos-Luci et al.
European journal of medicinal chemistry, 53, 264-274 (2012-05-09)
In this study, we describe the synthesis of a series of α- and β-lapachone containing hydroxyl or methoxyl groups on the benzene ring, by means of the selective acid promoted cyclization of the appropriate lapachol analog. The evaluation of the
Dong-Oh Moon et al.
International immunopharmacology, 7(4), 506-514 (2007-02-27)
beta-Lapachone (LAPA) is a chemotherapeutic agent that can inhibit the expression of nitric oxide (NO) and inducible NO synthase (iNOS) in alveolar macrophages. No other information on the agent's anti-inflammatory activity has been reported. In the present study, we investigated
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service