133337
4-Acetamidobenzoic acid
98%
Synonym(s):
N-Acetyl-PABA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
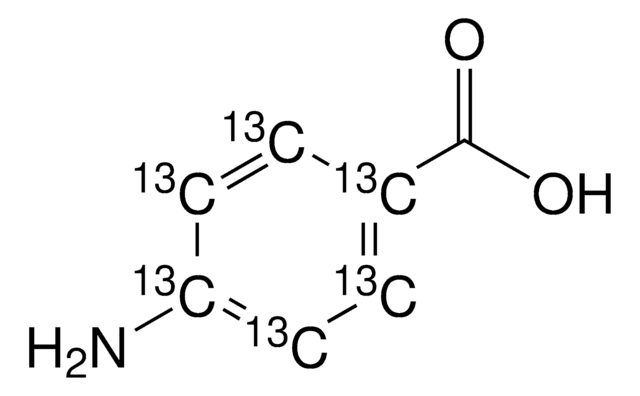
CH3CONHC6H4CO2H
CAS Number:
Molecular Weight:
179.17
Beilstein:
390602
EC Number:
MDL number:
UNSPSC Code:
12352106
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
259-262 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
CC(=O)Nc1ccc(cc1)C(O)=O
InChI
1S/C9H9NO3/c1-6(11)10-8-4-2-7(3-5-8)9(12)13/h2-5H,1H3,(H,10,11)(H,12,13)
InChI key
QCXJEYYXVJIFCE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
João Neres et al.
Bioorganic & medicinal chemistry, 15(5), 2106-2119 (2007-01-16)
Benzoic acid and pyridine derivatives inhibit recombinant trans-sialidase from Trypanosoma cruzi with I50 values between 0.4 and 1mM. The best compounds, 4-acetylamino-3-hydroxymethylbenzoic acid and 5-acetylamino-6-aminopyridine-2-carboxylic acid, provide new leads to inhibitors not containing the synthetically complex sialic acid structure. The
K N Furuya et al.
Clinical biochemistry, 28(5), 531-540 (1995-10-01)
To evaluate glycine conjugation of para-aminobenzoic acid (PABA) to the hippurated metabolites, para-aminohippuric acid (PAHA), and para-acetamidohippuric acid (PAAHA) as a quantitative liver function test in patients with liver disease. Serum concentrations of PABA and metabolites were measured by high
In vitro histamine release induced by radiocontrast media and various chemical analogs in reactor and control subjects.
M C Rice et al.
The Journal of allergy and clinical immunology, 72(2), 180-186 (1983-08-01)
Reversed-phase high-performance liquid chromatographic assay for the determination of the in vitro acetylation of p-aminobenzoic acid by human whole blood.
R M Lindsay et al.
Journal of chromatography, 433, 292-297 (1988-12-09)
B Barbieri et al.
Thrombosis research, 95(5), 235-243 (1999-10-09)
We have previously found that the naturally occurring amine p-aminobenzoic acid (PABA) inhibits the thrombin-induced thromboxane B2 production in human platelets. In this report we show that PABA and its acetylated metabolite p-acetamidobenzoic acid (PACBA) inhibit platelet aggregation induced by
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service