125954
Dichloroacetonitrile
98%
Synonym(s):
2,2-Dichloroacetonitrile, Dichloromethyl cyanide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
Cl2CHCN
CAS Number:
Molecular Weight:
109.94
Beilstein:
1739029
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.44 (lit.)
bp
110-112 °C (lit.)
density
1.369 g/mL at 25 °C (lit.)
SMILES string
ClC(Cl)C#N
InChI
1S/C2HCl2N/c3-2(4)1-5/h2H
InChI key
STZZWJCGRKXEFF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Dichloroacetonitrile can be used as a reactant to prepare:
- Chiral α, α-dichloro-β-aminonitriles via Pd-catalyzed enantioselective Mannich-type reaction with imines.
- α, α-dialkyl-substituted nitriles by an alkylation reaction with trialkylboranes in the presence of phenoxide base as a base.
- Halogenated pyridines via copper-catalyzed reaction with methacrolein.
- α,α-dichloro-β-hydroxy nitriles by condensation reaction with aldehydes and ketones in the presence of an alkoxide base.
- Selenium heterocycle derivatives via Diels–Alder cyclization with selenoaldehydes.
- Dichloroacetonitrile can also be used to develop an efficient method for the extraction and determination of common volatile halogenated disinfection by-products using the static headspace technique coupled with gas chromatography-mass spectrometry.
Biochem/physiol Actions
Dichloroacetonitrile is direct-acting mutagen and induces DNA strand breaks in cultured human lymphoblastic cells. It induces apoptosis or necrosis in murine macrophage cell line via reactive oxygen intermediates-mediated oxidative mechanisms of cellular damage.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Flam. Liq. 3 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
96.8 °F - closed cup
Flash Point(C)
36 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Speciation of common volatile halogenated disinfection by-products in tap water under different oxidising agents
Montesinos I and Gallego M
Journal of Chromatography A, 1310, 113-120 (2013)
M K Smith et al.
Fundamental and applied toxicology : official journal of the Society of Toxicology, 12(4), 765-772 (1989-05-01)
Dichloroacetonitrile (DCAN), a by-product of drinking water disinfection formed by reaction of chlorine with background organic materials, was evaluated for its developmental effects in pregnant Long-Evans rats. Animals were dosed by oral intubation on Gestation Days 6-18 (plug = 0)
Dichloroacetonitrile
GL Bundy
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
Isabel Montesinos et al.
Journal of chromatography. A, 1310, 113-120 (2013-09-03)
A simple and efficient method has been developed for the extraction and determination of sixteen common volatile halogenated disinfection by-products (DBPs) using the static headspace (HS) technique coupled with gas chromatography-mass spectrometry (GC-MS). The DBPs determined included trihalomethanes (THMs), halonitromethanes
Huang Huang et al.
Environmental science & technology, 46(19), 10624-10631 (2012-09-07)
The increasing usage of organic nitrogen-rich wastewater- or algal-impacted waters, and chloramines for secondary disinfection, raises concerns regarding the formation of haloacetonitriles, haloacetamides and other nitrogenous disinfection byproducts (N-DBPs). Previous research obtained contradictory results regarding the relative importance of chlorination
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
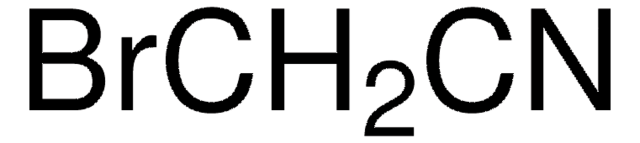





![Bicyclo[2.2.1]hept-2-ene 99%](/deepweb/assets/sigmaaldrich/product/structures/270/492/95fd4909-6108-4858-8c94-609b54387149/640/95fd4909-6108-4858-8c94-609b54387149.png)



