All Photos(2)
About This Item
Linear Formula:
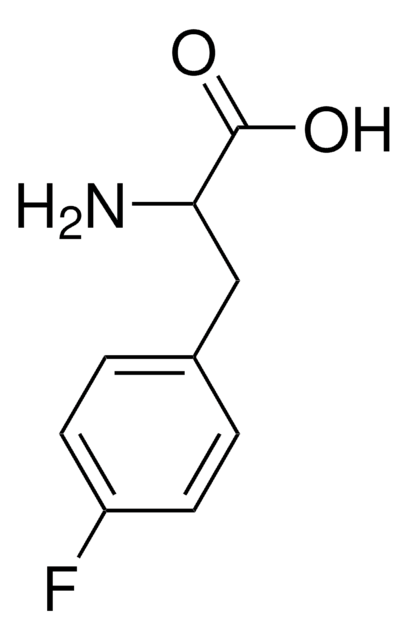
BrC6H4CH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
244.09
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
mp
262-263 °C (dec.) (lit.)
application(s)
peptide synthesis
storage temp.
2-8°C
SMILES string
NC(Cc1ccc(Br)cc1)C(O)=O
InChI
1S/C9H10BrNO2/c10-7-3-1-6(2-4-7)5-8(11)9(12)13/h1-4,8H,5,11H2,(H,12,13)
InChI key
PEMUHKUIQHFMTH-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dose fractionation in neutron capture therapy for malignant melanoma.
B J Allen et al.
Basic life sciences, 50, 63-67 (1989-01-01)
G Basu et al.
Biochemistry, 32(12), 3067-3076 (1993-03-30)
The very strong helical propensity of peptides rich in alpha-aminoisobutyric acid (Aib) has enabled the design of a set of helices containing as guest amino acids one fluorescent chromophore, beta-(1'-naphthyl)-L-alanine, and one heavy atom perturber, p-bromo-L-phenylalanine. The fluorescence of the
James M Turner et al.
Proceedings of the National Academy of Sciences of the United States of America, 103(17), 6483-6488 (2006-04-19)
Recently, tRNA aminoacyl-tRNA synthetase pairs have been evolved that allow one to genetically encode a large array of unnatural amino acids in both prokaryotic and eukaryotic organisms. We have determined the crystal structures of two substrate-bound Methanococcus jannaschii tyrosyl aminoacyl-tRNA
M Ibba et al.
FEBS letters, 364(3), 272-275 (1995-05-15)
It has previously been demonstrated that the unnatural amino acid p-Cl-phenylalanine can be attached to tRNA(Phe) by a modified phenylalanyl-tRNA synthetase with relaxed amino acid substrate specificity. We show that this modification to the translational machinery of Escherichia coli is
Shun Zheng et al.
Biotechnology and bioengineering, 110(9), 2361-2370 (2013-04-10)
Enzyme inhibition plays an important role in drug development, metabolic pathway regulation, and biocatalysis with product inhibition. When an inhibitor has high structural similarities to the substrate of an enzyme, controlling inhibitor binding without affecting enzyme substrate binding is often
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service