656364
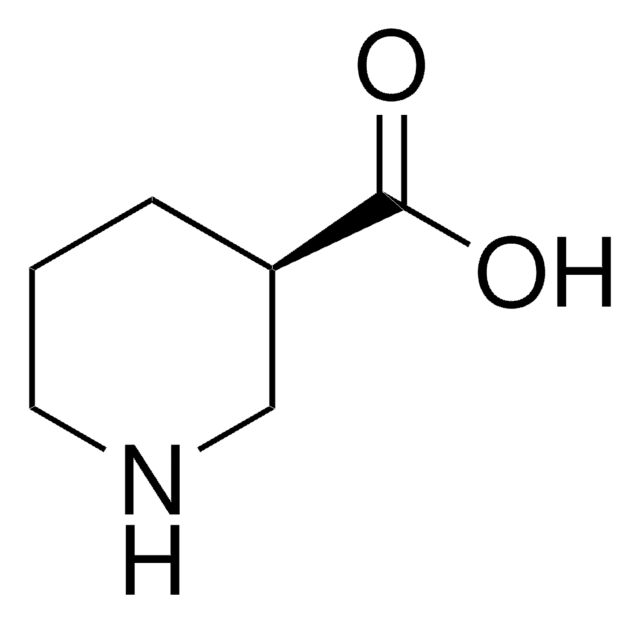
(S)-(+)-3-Piperidinecarboxylic acid
97%
Synonym(s):
(S)-(+)-Nipecotic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H11NO2
CAS Number:
Molecular Weight:
129.16
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
optical activity
[α]/D 3.0 to 6.5°, c = 1 in H2O
mp
254 °C (dec.)
functional group
carboxylic acid
SMILES string
OC(=O)[C@H]1CCCNC1
InChI
1S/C6H11NO2/c8-6(9)5-2-1-3-7-4-5/h5,7H,1-4H2,(H,8,9)/t5-/m0/s1
InChI key
XJLSEXAGTJCILF-YFKPBYRVSA-N
Gene Information
rat ... Slc6a1(79212)
Looking for similar products? Visit Product Comparison Guide
General description
(S)-(-)-3-Piperidinecarboxylic acid is an inhibitor of GABA (γ-aminobutyric acid) uptake.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Anna-Lucia Koerling et al.
Neuropharmacology, 148, 394-405 (2018-11-26)
In addition to reducing seizures, anti-epileptic treatments should preserve physiological network activity. Here, we used a thalamocortical slice preparation displaying physiological slow oscillations to investigate the effects of anticonvulsant drugs on physiological activity and epileptiform activity in two pharmacological epilepsy
Hong Gao et al.
Neuropharmacology, 58(8), 1220-1227 (2010-03-17)
Zolpidem is a widely prescribed sleep aid with relative selectivity for GABA(A) receptors containing alpha1-3 subunits. We examined the effects of zolpidem on the inhibitory currents mediated by GABA(A) receptors using whole-cell patch-clamp recordings from DMV neurons in transverse brainstem
Michael P DeNinno et al.
Bioorganic & medicinal chemistry letters, 21(10), 3095-3098 (2011-04-05)
The first highly potent and selective PDE8 inhibitors are disclosed. The initial tetrahydroisoquinoline hit was transformed into a nipecotic amide series in order to address a reactive metabolite issue. Reduction of lipophilicity to address metabolic liabilities uncovered an interesting diastereomer-dependent
Moslem Mohammadi et al.
Toxicology, 244(1), 42-48 (2007-12-07)
A synaptosomal model was used to evaluate in vivo effects of paraoxon on the uptake of [(3)H]GABA in rat cerebral cortex and hippocampus. Male Wistar rats were given a single intraperitoneal injection of one of three doses of paraoxon (0.1
Inhibition of the uptake of GABA and related amino acids in rat brain slices by the optical isomers of nipecotic acid.
G A Johnston et al.
Journal of neurochemistry, 26(5), 1029-1032 (1976-05-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service