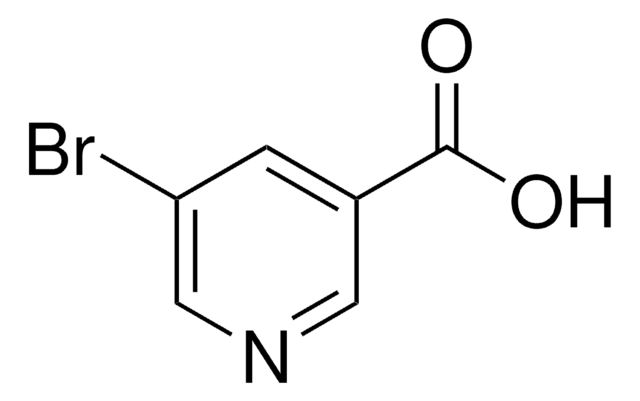
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C5H3BrN4O
CAS Number:
Molecular Weight:
215.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
mp
>350 °C (lit.)
functional group
bromo
SMILES string
BrC1=Nc2nc[nH]c2C(=O)N1
InChI
1S/C5H3BrN4O/c6-5-9-3-2(4(11)10-5)7-1-8-3/h1H,(H2,7,8,9,10,11)
InChI key
ONXCBJOMYNPZNI-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
John M Caddell et al.
The Journal of organic chemistry, 69(9), 3212-3215 (2004-04-24)
A convergent synthesis of adenosine A2a agonist 1 in the form of its maleate salt 2 was achieved. The key step in this approach was the highly selective 9beta-glycosylation reaction between 2-haloadenines or an N(2)-alkyl-6-chloroguanine and a D-ribose derivative containing
M M Butler et al.
Nucleic acids research, 18(24), 7381-7387 (1990-12-25)
6-(p-Hydroxyphenylhydrazino)uracil (H2-HPUra) is a selective and potent inhibitor of the replication-specific class III DNA polymerase (pol III) of Gr+ bacteria. Although formally a pyrimidine, H2-HPUra derives its inhibitory activity from its specific capacity to mimic the purine nucleotide, dGTP. We
Sheng Ding et al.
Journal of combinatorial chemistry, 4(2), 183-186 (2002-03-12)
A resin-capture and release strategy for making combinatorial 2,6,9-trisubstituted purine libraries is demonstrated by capturing N9-derivatized purines at the C6 position with a thio-modified polymer. The C2 fluoro group is subsequently substituted with primary and secondary amines followed by thioether
R S Sodum et al.
Chemical research in toxicology, 11(12), 1453-1459 (1998-12-22)
2-Nitropropane, an industrial chemical and a hepatocarcinogen in rats, induces aryl sulfotransferase-mediated liver DNA and RNA base modifications [Sodum, R. S., Sohn, O. S., Nie, G., and Fiala, E. S. (1994) Chem. Res. Toxicol. 7, 344-351]. Two of these modifications
Shin Nishiumi et al.
Journal of bioscience and bioengineering, 125(5), 613-618 (2017-12-21)
Recently, the use of metabolomic analysis of human serum and plasma for biomarker discovery and disease diagnosis in clinical studies has been increasing. The feasibility of using a metabolite biomarker for disease diagnosis is strongly dependent on the metabolite's stability
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service