308323
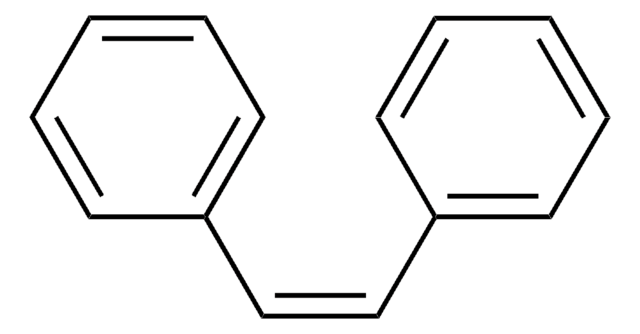
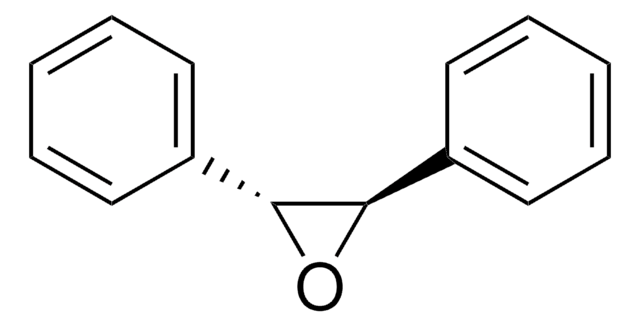
cis-Stilbene oxide
97%
Synonym(s):
cis-2,3-Diphenyloxirane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C14H12O
CAS Number:
Molecular Weight:
196.24
Beilstein:
82737
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Assay
97%
mp
38-40 °C (lit.)
storage temp.
2-8°C
SMILES string
O1[C@H]([C@H]1c2ccccc2)c3ccccc3
InChI
1S/C14H12O/c1-3-7-11(8-4-1)13-14(15-13)12-9-5-2-6-10-12/h1-10,13-14H/t13-,14+
InChI key
ARCJQKUWGAZPFX-OKILXGFUSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
228.2 °F - closed cup
Flash Point(C)
109 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Hesam Arabnejad et al.
Chembiochem : a European journal of chemical biology, 21(13), 1893-1904 (2020-01-22)
The use of enzymes in preparative biocatalysis often requires tailoring enzyme selectivity by protein engineering. Herein we explore the use of computational library design and molecular dynamics simulations to create variants of limonene epoxide hydrolase that produce enantiomeric diols from
Paloma Vidal et al.
The Journal of organic chemistry, 72(9), 3166-3170 (2007-03-10)
This study presents a simple method for measuring long-range heteronuclear coupling constants between protons and proton-bearing carbons. The approach involves recording two conventional 1D-TOCSY experiments in which the offset of the selective proton pulse is set on the low- and
B Schilter et al.
The Journal of pharmacology and experimental therapeutics, 294(3), 916-922 (2000-08-17)
Oxidative biotransformation, coupled with genetic variability in enzyme expression, has been the focus of hypotheses interrelating environmental and genetic factors in the etiology of central nervous system disease processes. Chemical modulation of cerebral cytochrome P450 (P450) monooxygenase expression character may
S Bernardini et al.
Mutagenesis, 16(3), 277-281 (2001-04-26)
About 50% and 15% of Caucasians lack the glutathione S-transferase M1 (GSTM1) and T1 (GSTT1) genes and the corresponding enzyme activity, respectively. Both of these polymorphisms have been shown to affect the genotoxicity of some epoxides in cultured human lymphocytes.
Ylva Ivarsson et al.
Biochimica et biophysica acta, 1770(9), 1374-1381 (2007-08-11)
Based on the crystal structure of human glutathione transferase M1-1, cysteine residues were introduced in the substrate-binding site of a Cys-free mutant of the enzyme, which were subsequently alkylated with 1-iodoalkanes. By different combinations of site-specific mutations and chemical modifications
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service