All Photos(2)
About This Item
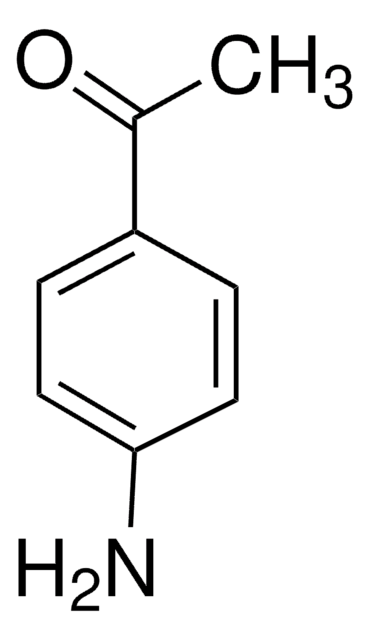
Linear Formula:
H2NC6H4CONH2
CAS Number:
Molecular Weight:
136.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
solid
mp
181-183 °C (lit.)
SMILES string
NC(=O)c1ccc(N)cc1
InChI
1S/C7H8N2O/c8-6-3-1-5(2-4-6)7(9)10/h1-4H,8H2,(H2,9,10)
InChI key
QIKYZXDTTPVVAC-UHFFFAOYSA-N
Related Categories
Application
4-Aminobenzamide was used as a poly(ADP-ribose)polymerase (PADPRP) inhhibitor to study the death of target cells by cytotoxic effector cells using the nuclear enzyme poly(ADP-ribose)polymerase (PADPRP).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Kinetics of heparin action.
J D Shore et al.
Annals of the New York Academy of Sciences, 556, 75-80 (1989-01-01)
R J Baugh et al.
The Journal of biological chemistry, 275(37), 28826-28833 (2000-07-13)
The initiation of coagulation results from the activation of factor X by an enzyme complex (Xase) composed of the trypsin-like serine proteinase, factor VIIa, bound to tissue factor (TF) on phospholipid membranes. We have investigated the basis for the protein
S D Ray et al.
Molecular and cellular biochemistry, 218(1-2), 27-33 (2001-05-02)
Previous studies from our laboratories have linked the protective abilities of IH636 grape seed proanthocyanidin extract (GSPE) with inactivation of anti-apoptotic gene bcl-XL, and modification of several other critical molecular targets such as DNA-damage/DNA-repair, lipid peroxidation and intracellular Ca2+ homeostasis.
Inhibition of nitric oxide induced cytotoxicity in pancreatic b-cells.
S A Brennan et al.
Biochemical Society transactions, 24(1), 73S-73S (1996-02-01)
S D Ray et al.
Free radical biology & medicine, 31(3), 277-291 (2001-07-20)
Acetaminophen (AAP), the analgesic hepatotoxicant, is a powerful inducer of oxidative stress, DNA fragmentation, and apoptosis. The anti-apoptotic oncogene bcl-XL, and the pro-apoptotic oncogene p53 are two key regulators of cell cycle progression and/or apoptosis subsequent to DNA damage in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service