G9043
Anti-GRP78/BiP (ET-21) antibody produced in rabbit

IgG fraction of antiserum, buffered aqueous solution
Synonym(s):
Anti-78-kDa Glucose-Regulating Protein, Anti-Immunoglobulin Heavy Chain Binding Protein
About This Item
Recommended Products
biological source
rabbit
conjugate
unconjugated
antibody form
IgG fraction of antiserum
antibody product type
primary antibodies
clone
polyclonal
form
buffered aqueous solution
mol wt
antigen 78 kDa
species reactivity
mouse, chicken, Xenopus, hamster, human, rat
enhanced validation
independent
Learn more about Antibody Enhanced Validation
technique(s)
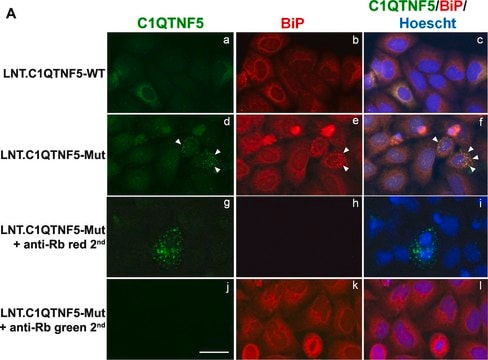
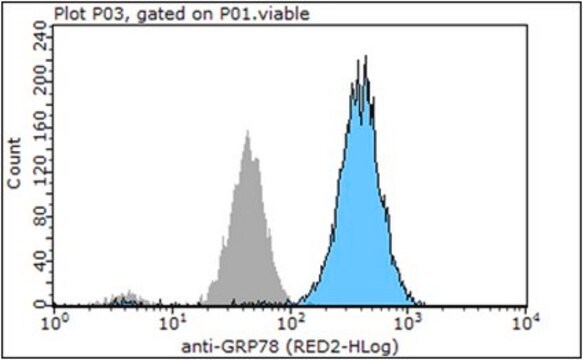
immunocytochemistry: 1:1,000 using methanol/acetone fixed cultured mouse fibroblast NIH3T3 cell line
immunohistochemistry: 1:1,000 using human cerebral cortex and human thyroid gland tissue sections
western blot: 1:3,000 using whole cell extract of human epitheloid carcinoma HeLa cell line
UniProt accession no.
shipped in
dry ice
storage temp.
−20°C
target post-translational modification
unmodified
Gene Information
human ... HSPA5(3309)
mouse ... Hspa5(14828)
rat ... Hspa5(25617)
Application
- for immunofluorescence staining of frozen heart tissue to examine whether ER stress signaling activation occurs in cardiomyocytes
- for immunohistochemistry on Paraffin embedded sections of human cerebral cortex and human thyroid gland tissue sections.
- to detect Bip by immunoblotting of protein extract from WT AB strain of zebrafish (Danio rerio)
- at a working dilution of 1:1000 to detect GRP78 in extract of prostate cancer cells after androgen deprivation therapy
Biochem/physiol Actions
Physical form
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Articles
Centrifugation separates organelles based on size, shape, and density, facilitating subcellular fractionation across various samples.
Centrifugation separates organelles based on size, shape, and density, facilitating subcellular fractionation across various samples.
Centrifugation separates organelles based on size, shape, and density, facilitating subcellular fractionation across various samples.
Centrifugation separates organelles based on size, shape, and density, facilitating subcellular fractionation across various samples.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service