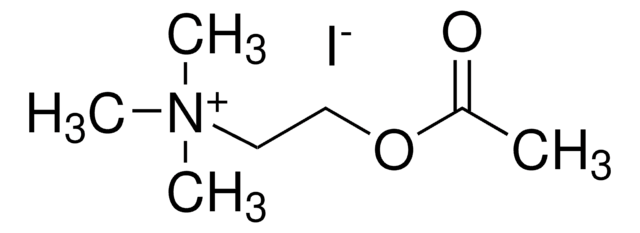
B3253
Butyrylthiocholine iodide
≥98%
Synonym(s):
(2-Mercaptoethyl)trimethylammonium iodide butyrate
About This Item
Recommended Products
Quality Level
Assay
≥98%
form
powder
mp
171-174 °C (lit.)
storage temp.
−20°C
SMILES string
[I-].CCCC(=O)SCC[N+](C)(C)C
InChI
1S/C9H20NOS.HI/c1-5-6-9(11)12-8-7-10(2,3)4;/h5-8H2,1-4H3;1H/q+1;/p-1
InChI key
WEQAAFZDJROSBF-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Label-Free and Ultrasensitive Detection of Butyrylcholinesterase: A study demonstrated the use of Mn(II)-based electron spin resonance spectroscopy for the ultrasensitive detection of butyrylcholinesterase, using Butyrylthiocholine iodide as a substrate to quantify enzyme activity in the presence of organophosphorus pesticides, crucial for biochemical assay applications (Tang et al., 2022).
- Novel Nanozyme for Biosensing: Research developed a Co, N co-doped porous carbon-based nanozyme, demonstrating its utility as an oxidase mimic for fluorescence and colorimetric biosensing of butyrylcholinesterase, employing Butyrylthiocholine iodide as a key substrate, relevant in enzyme kinetics analysis (Sun et al., 2022).
- Detection System for Anti-Alzheimer′s Drug Screening: A fluorescent platform was constructed using copper nanoclusters and MnO2 nanosheets for the detection of butyrylcholinesterase activity, utilizing Butyrylthiocholine iodide, which may facilitate the screening of anti-Alzheimer′s drugs and probe cholinergic system interactions (Chen et al., 2022).
- Dual-Channel Detection of Butyrylcholinesterase: A study introduced bifunctional metal-organic frameworks with integrated fluorescence and oxidase activities, developed for dual-channel detection of butyrylcholinesterase using Butyrylthiocholine iodide, enhancing methodologies in biochemical assays (Wang et al., 2022).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service