852473
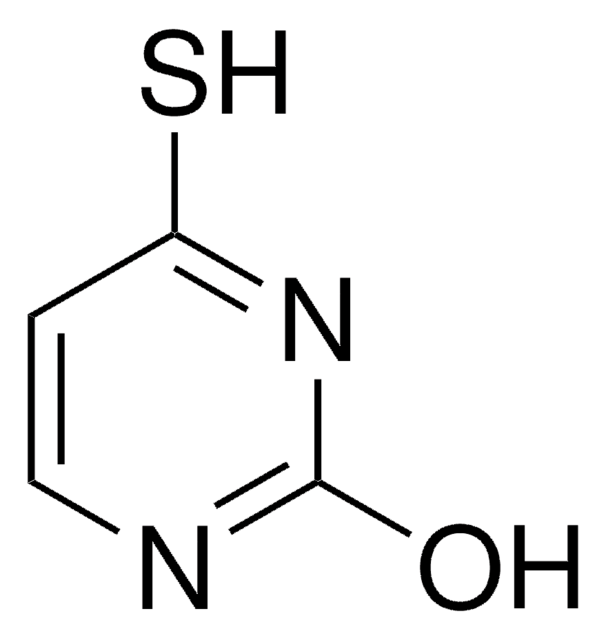
5-Bromouracil
98%
Synonym(s):
5-Bromo-2,4-dihydroxypyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C4H3BrN2O2
CAS Number:
Molecular Weight:
190.98
Beilstein:
127176
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
crystals
mp
>300 °C (lit.)
SMILES string
BrC1=CNC(=O)NC1=O
InChI
1S/C4H3BrN2O2/c5-2-1-6-4(9)7-3(2)8/h1H,(H2,6,7,8,9)
InChI key
LQLQRFGHAALLLE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
E Itälä et al.
The Journal of chemical physics, 133(15), 154316-154316 (2010-10-26)
Photofragmentation of thymine and 5-bromouracil into cation and neutral fragments following the core ionization by soft x-rays using photoelectron-photoion-photoion coincidence technique has been studied. The fragment ion mass spectra were recorded in coincidence with the C 1s photoelectron spectra. In
Zejun Li et al.
The journal of physical chemistry. B, 115(46), 13668-13673 (2011-09-10)
The reaction of low-energy electrons (LEEs; 10 eV) with 5'-TpXpT-3' (TXT), where X is uracil (U), thymine (T), and 5-bromouracil (5BrU), was examined by HPLC-UV analysis. The presence of 5BrU increased total damage by >50%. The radiation products of T5BrUT
Pierre Daublain et al.
The journal of physical chemistry. B, 114(45), 14265-14272 (2010-02-04)
The photophysical and photochemical behavior of a series of hairpin-forming DNA conjugates possessing a 5'-tethered pyrenecarboxamide chromophore and one or two bromouracil bases has been investigated. Quenching of the pyrene fluorescence and transient absorption spectra characteristic of the pyrene cation
Hironobu Morinaga et al.
Bioorganic & medicinal chemistry, 21(2), 466-469 (2012-12-26)
5-Bromouracil ((Br)U) was incorporated into three types of synthetic RNA and the products of the photoirradiated (Br)U-containing RNAs were investigated using HPLC and MS analysis. The photoirradiation of r(GCA(Br)UGC)(2) and r(CGAA(Br)UUGC)/r(GCAAUUCG) in A-form RNA produced the corresponding 2'-keto adenosine ((keto)A)
The lesions produced by ultraviolet light in DNA containing 5-bromouracil.
F Hutchinson
Quarterly reviews of biophysics, 6(2), 201-246 (1973-05-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service