744867
(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate
for Copper-free Click Chemistry
Synonym(s):
N-[((1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-yl)methyloxycarbonyloxy]succinimide, BCN-NHS, BCN-succinimidyl ester
About This Item
Recommended Products
form
powder
composition
carbon content, 61.85%
hydrogen content, 5.88%
nitrogen content, 4.81%
reaction suitability
reaction type: click chemistry
reagent type: cross-linking reagent
functional group
NHS ester
storage temp.
−20°C
SMILES string
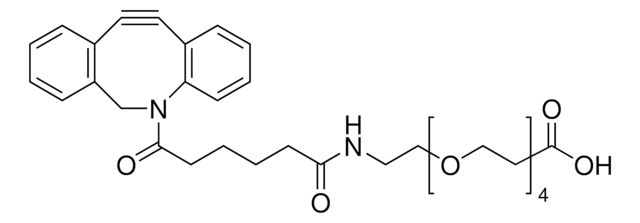
[H][C@@]12CCC#CCC[C@]1([H])[C@@H]2COC(ON3C(CCC3=O)=O)=O
InChI
1S/C15H17NO5/c17-13-7-8-14(18)16(13)21-15(19)20-9-12-10-5-3-1-2-4-6-11(10)12/h10-12H,3-9H2/t10-,11+,12-
InChI key
SKTDJYHCSCYLQU-ZSBIGDGJSA-N
Application
It may also be used to synthesize bicyclononyne functionalized poly(ethylene glycol) polymer coatings with anti-fouling properties towards protein adhesion and cell adhesion for supramolecular ureidopyrimidinone (UPy) based materials.
Packaging
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Copper-free click chemistry is an alternative approach to click chemistry that proceeds at a lower activation barrier and is free of cytotoxic transition metal catalysts.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethanol for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/171/632/0556139a-2db5-4678-a6ec-a26a693fd574/640/0556139a-2db5-4678-a6ec-a26a693fd574.png)

![N-[(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyloxycarbonyl]-1,8-diamino-3,6-dioxaoctane for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/294/853/c5e47d84-5aee-4797-aa24-604f291171cc/640/c5e47d84-5aee-4797-aa24-604f291171cc.png)