456128
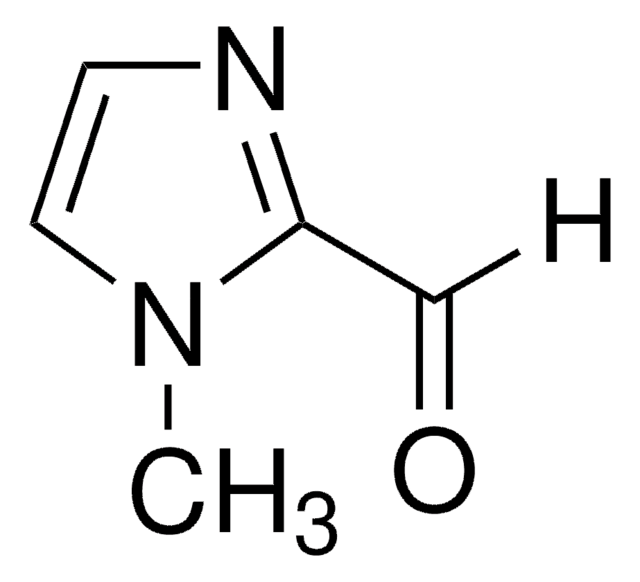
4-Imidazolecarboxaldehyde
98%
Synonym(s):
5-Imidazolecarboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C4H4N2O
CAS Number:
Molecular Weight:
96.09
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
174-177 °C (lit.)
SMILES string
O=Cc1c[nH]cn1
InChI
1S/C4H4N2O/c7-2-4-1-5-3-6-4/h1-3H,(H,5,6)
InChI key
ZQEXIXXJFSQPNA-UHFFFAOYSA-N
General description
4-Imidazolecarboxaldehyde undergoes reductive amination with the amine in the presence of sodium borohydride to form secondary amines.
Application
4-Imidazolecarboxaldehyde may be used in the following studies:
- Synthesis of new donor-Π-acceptor (D-Π-A) type dye.
- Preparation of ethyl, n-dodecyl and n-hexadecyl esters of urocanic acid (4-imidazoleacrylic acid).
- Fabrication of colorimetric chemosensor.
- Synthesis of chiral nickel(II) complex, [Ni(II)H3L](ClO4)2, having an achiral ligand H3L (tris{2-(4-imidazolyl)methyliminoethyl}amine).
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Spontaneous Resolution Induced by a Chiral Ni (II) Complex with an Achiral Tripodal Ligand#.
Sarkar S, et al.
Bull. Korean Chem. Soc., 36(3), 838-842 (2015)
Peter B Madrid et al.
Journal of combinatorial chemistry, 6(3), 437-442 (2004-05-11)
Due to growing problems with drug resistance, there is an outstanding need for new, cost-effective drugs for the treatment of malaria. The 4-aminoquinolines have provided a number of useful antimalarials, and Plasmodium falciparum, the causative organism for the most deadly
Organic Process Research & Development, 11, 206-206 (2007)
Chromene and Imidazole Based D-p-A Chemosensor Preparation and Its Anion Responsive Effects.
Son YA, et al.
Mol. Cryst. Liq. Cryst., 599(1), 16-22 (2014)
Dongli Wang et al.
The journal of gene medicine, 22(10), e3240-e3240 (2020-06-20)
Gene therapy has become a potential strategy for cancer treatment. However, the development of efficient gene vectors restricts the application for cancer gene treatment. Functionalization of polymers with functional groups can significantly improve their transfection efficacy. Guanidyl can form bidentate
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service