All Photos(2)
About This Item
Empirical Formula (Hill Notation):
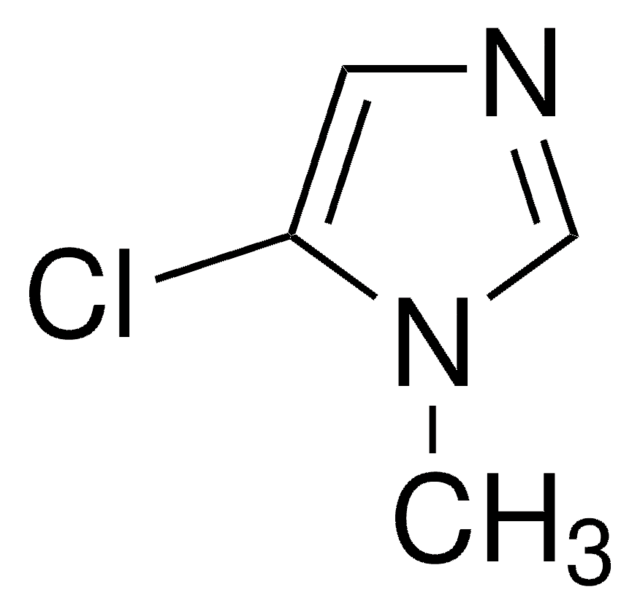
C4H4ClN3O2
CAS Number:
Molecular Weight:
161.55
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
148-150 °C (lit.)
SMILES string
Cn1cnc(c1Cl)[N+]([O-])=O
InChI
1S/C4H4ClN3O2/c1-7-2-6-4(3(7)5)8(9)10/h2H,1H3
InChI key
OSJUNMSWBBOTQU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5-Chloro-1-methyl-4-nitroimidazole is an 4-nitroimidazole derivative.
Application
5-Chloro-1-methyl-4-nitroimidazole (CMNI) is suitable for use in the rapid mix experiments to investigate the mechanism of anomalous radiosensitization of mammalian cells by CMNI. It may be used in the synthesis of 5-aryl-1-methyl-4-nitroimidazoles, via Suzuki coupling with arylboronic acids, catalyzed by dichlorobis-(triphenylphosphine)palladium(II), K2CO3 and tetrabutylammonium bromide.
related product
Product No.
Description
Pricing
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rapid-mix studies on the anomalous radiosensitization of mammalian cells by 5-chloro-1-methyl-4-nitromidazole.
M E Watts et al.
International journal of radiation biology and related studies in physics, chemistry, and medicine, 38(6), 673-675 (1980-12-01)
J Watras et al.
Neoplasma, 34(3), 253-259 (1987-01-01)
The transplantable rhabdomyosarcoma in WAG/Rij rats was used to test the in vivo effectiveness of 1-methyl-2-chloro-4-nitroimidazole (P13) and its analog 1-(2-hydroxy-3-methoxy-propyl)-2-chloro-4-nitroimidazole (P40) as tumor-cell radiosensitizers after their i.p. administration at low doses. The results indicate that both compounds administered repeatedly
Are ortho-substituted 4-nitroimidazoles a new generation of radiation-induced arylating agents?
E D Clarke et al.
International journal of radiation biology and related studies in physics, chemistry, and medicine, 37(4), 463-468 (1980-04-01)
Occupational allergic contact dermatitis from 5-chloro-1-methyl-4-nitroimidazole.
R Jolanki et al.
Contact dermatitis, 36(1), 53-54 (1997-01-01)
The kinetics of the reaction of 'anomalous' 4-nitroimidazole radiosensitizers with thiols.
P Wardman
International journal of radiation biology and related studies in physics, chemistry, and medicine, 41(2), 231-235 (1982-02-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service