348015
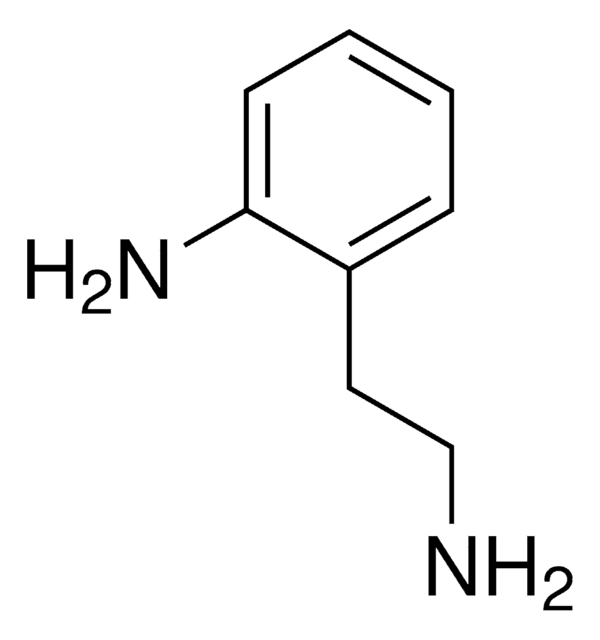
2-Aminobenzylamine
98%
Synonym(s):
(2-Aminomethylphenyl)amine, 2-(Aminomethyl)aniline, 2-(Aminomethyl)benzenamine, 2-Amino-1-benzylamine, [(2-Aminophenyl)methyl]amine, o-Aminobenzylamine
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2NC6H4CH2NH2
CAS Number:
Molecular Weight:
122.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
solid
mp
58-61 °C (lit.)
functional group
amine
SMILES string
NCc1ccccc1N
InChI
1S/C7H10N2/c8-5-6-3-1-2-4-7(6)9/h1-4H,5,8-9H2
InChI key
GVOYKJPMUUJXBS-UHFFFAOYSA-N
General description
2-Aminobenzylamine undergoes three-component cyclisation reactions with methyl 3,3,3-trifluoropyruvate, 2-aminobenzylamine and oxo compounds to afford regio- and stereoisomers of tetrahydropyrroloquinazolinones.
Application
2-Aminobenzylamine may be used:
- in the synthesis of 1,2,3,4-tetrahydroquinazoline oxime, via condensation reaction with 2-(naphthalen-2-yl)-2-oxoacetaldehyde oxime
- in the synthesis of alkyl 5H-1,4-benzodiazepine-3-carboxylates
- to modify the phosphate groups on phosphoserine peptides
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
G Sirikçi et al.
Acta chimica Slovenica, 59(4), 904-911 (2013-09-26)
A novel 1,2,3,4-tetrahydroquinazoline oxime was synthesised from a condensation reaction of 2-(naphthalen-2-yl)-2-oxoacetaldehyde oxime with 2-aminobenzylamine. Subsequently, a-imine oxime complexes of this compound that formed with Co(III) and Ni(II) metal ions were obtained. All structures were characterised by spectral methods (FT-IR
Wen-Yun Hsueh et al.
Journal of medicinal chemistry, 64(3), 1435-1453 (2021-01-26)
In this paper, we present a copper(I)-catalyzed nitrile-addition/N-arylation ring-closure cascade for the synthesis of 5,11-dihydro-6H-indolo[3,2-c]quinolin-6-ones from 2-(2-bromophenyl)-N-(2-cyanophenyl)acetamides. Using CuBr and t-BuONa in dimethylformamide (DMF) as the optimal reaction conditions, the cascade reaction gave the target products, in high yields, with
Bohumil Dolenský et al.
Magnetic resonance in chemistry : MRC, 48(5), 375-385 (2010-03-20)
A new three-component cyclisation reactions of methyl 3,3,3-trifluoropyruvate, 2-aminobenzylamine and oxo compounds afforded tetrahydropyrroloquinazolinones of the types 4 and 5 as mixtures of regio- and stereoisomers. Whereas standard 1D NMR spectroscopy was used for a facile assignment of the cyclization
Lukas K Filak et al.
Organometallics, 32(3), 903-914 (2013-02-23)
Six novel ruthenium(II)- and osmium(II)-arene complexes with three modified indolo[3,2-c]quinolines have been synthesized in situ starting from 2-aminoindoloquinolines and 2-pyridinecarboxaldehyde in the presence of [M(p-cymene)Cl(2)](2) (M = Ru, Os) in ethanol. All complexes have been characterized by elemental analysis, spectroscopic
Lukas K Filak et al.
Organometallics, 30(2), 273-283 (2011-01-22)
The synthesis of new modified indolo[3,2-c]quinoline ligands L(1)-L(8) with metal-binding sites is reported. By coordination to ruthenium- and osmium-arene moieties 16 complexes of the type [(η(6)-p-cymene)M(L)Cl]Cl (1a,b-8a,b), where M is Ru(II) or Os(II) and L is L(1)-L(8), have been prepared.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service