105171
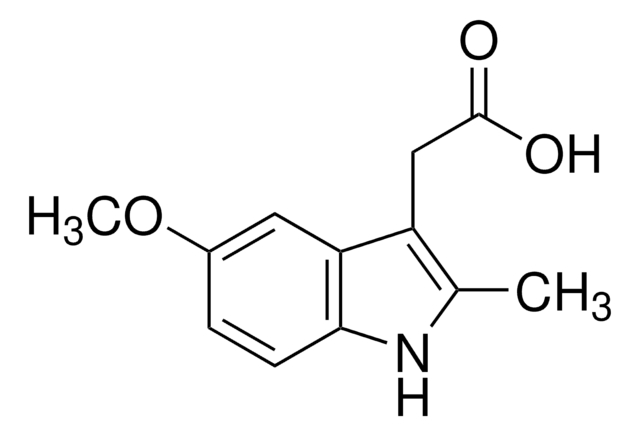
5-Methoxy-2-methyl-3-indoleacetic acid
98%
Synonym(s):
N-Des(4-chlorobenzoyl)indomethacin, NSC 97026, 2-(5-Methoxy-2-methyl-1H-indol-3-yl)acetic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C12H13NO3
CAS Number:
Molecular Weight:
219.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
161-163 °C (lit.)
solubility
methanol: soluble
functional group
carboxylic acid
SMILES string
COc1ccc2[nH]c(C)c(CC(O)=O)c2c1
InChI
1S/C12H13NO3/c1-7-9(6-12(14)15)10-5-8(16-2)3-4-11(10)13-7/h3-5,13H,6H2,1-2H3,(H,14,15)
InChI key
TXWGINUZLBAKDF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
5-Methoxy-2-methyl-3-indoleacetic acid is hydrolysis product of indomethacin.
Application
5-Methoxy-2-methyl-3-indoleacetic acid was used for quantitative determination of indomethacin and its major impurities in suppository and capsule formulations by HPLC. 5-Methoxy-2-methyl-3-indoleacetic acid was used in a study to develop fast, sensitive and simultaneous determination of metabolites of serotonin using liquid chromatography with mass spectrometric detection.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
H Alho et al.
Cell growth & differentiation : the molecular biology journal of the American Association for Cancer Research, 5(9), 1005-1014 (1994-09-01)
A recognition site for benzodiazepines structurally different from that linked to the gamma-aminobutyric acid receptor subtype A or the "central type" benzodiazepine receptor has been located mainly in the outer membranes of mitochondria and designated mitochondrial benzodiazepine receptor (MBR). A
Peng Zhang et al.
Acta pharmacologica Sinica, 27(8), 1097-1102 (2006-07-27)
To investigate the biotransformation of indomethacin, the first of the newer nonsteroidal anti-inflammatory drugs, by filamentous fungus and to compare the similarities between microbial transformation and mammalian metabolism of indomethacin. Five strains of Cunninghamella (C elegans AS 3.156, C elegans
E Kwong et al.
Journal of pharmaceutical sciences, 71(7), 828-830 (1982-07-01)
Indomethacin and its impurities in suppository and capsule formulations were quantitatively determined by HPLC using a reversed-phase, octadecyl column and a mobile phase of methanol-water-acetonitrile-acetic acid (55:35:10:1). Analysis of the suppository formulations provided a mean potency for indomethacin of 103.8%.
P C Smith et al.
Journal of chromatography, 306, 315-321 (1984-03-09)
Quantitation of total amounts (i.e., free compound plus glucuronide conjugate) of indomethacin (INDO) and its deschlorobenzoyl (DBI) and desmethyl (DMI) metabolites in human urine is described. An aliquot (0.4 ml) of urine is incubated with glucuronidase (1000 U, 2 h
J Krzek et al.
Journal of AOAC International, 84(6), 1703-1707 (2002-01-05)
A densitometric method was developed for the identification and determination of indomethacin and its degradation products, 4-chlorobenzoic acid and 5-methoxy-2-methyl-3-indoleacetic acid, in pharmaceuticals. To separate these compounds, silica gel-coated thin-layer chromatography plates and the following mobile phase were used: 2-propanol-25%
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service