LC/MS Analysis of Omeprazole and Metabolites in Plasma on Ascentis® Express RP Amide after Sample Prep using HybridSPE®-Phospholipid
Materiales
analytical column
SPE tube or plate
CONDITIONS
sample preparation
SPE (Solid Phase Extraction)
sample/matrix
rat plasma spiked with omeprazole, phenacetin and metabolites at 2 μg/mL
SPE well plate
HybridSPE-Phospholipid, 96-well plate, 15 mg/0.8 mL (575656-U)
sample addition
100 μL plasma to each well, followed by 300 mL of 1% formic acid in acetonitrile
elution
apply vacuum
eluate post-treatment
eluate was collected, evaporated, and reconstituted in mobile phase.
column
Ascentis Express RP-Amide, 5 cm x 2.1 mm I.D., 2.7 μm particles (53911-U)
mobile phase
[A] water, 10 mM ammonium formate, pH 3.4; [B] methanol, 10 mM ammonium formate, pH 3.4; (65:35, A:B)
flow rate
0.3 mL/min
column temp.
35 °C
detector
ESI(+), m/z = 50-400
injection
1 μL
Descripción
Application
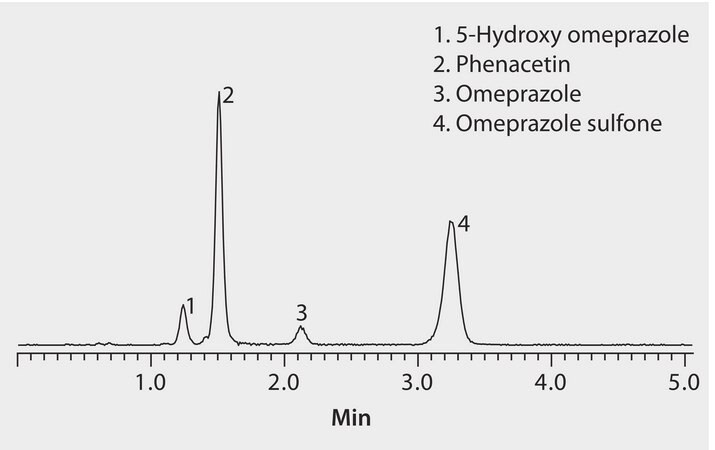
This application demonstrates the suitability of HybridSPE and the Ascentis Express RP Amide for the analysis of omeprazole metabolites.
Analysis Note
Sample was vorlexed and centrifuged for 2 minutes at 15000 rpm. Supemate was then passed through HybridSPE 96 well plate. Elutent was collected, evaporated and reconstituted in mobile phase.
Legal Information
Ascentis is a registered trademark of Merck KGaA, Darmstadt, Germany
HybridSPE is a registered trademark of Merck KGaA, Darmstadt, Germany