T1327
Tissue Inhibitor of Metalloproteinase-3 human
recombinant, expressed in NSO cells, >95% (SDS-PAGE)
Sinónimos:
TIMP-3
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Productos recomendados
origen biológico
human
Nivel de calidad
recombinante
expressed in NSO cells
Ensayo
>95% (SDS-PAGE)
Formulario
lyophilized powder
mol peso
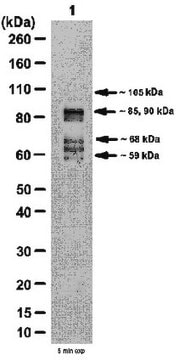
apparent mol wt ~30 kDa
técnicas
inhibition assay: suitable
Nº de acceso UniProt
temp. de almacenamiento
−20°C
Información sobre el gen
human ... TIMP3(7078)
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Tissue inhibitor of metalloproteinases 3 (TIMP3) is localized in the extracellular matrix (ECM) of epithelial cells associated with kidneys, eyes and lungs. It is majorly secreted in retinal pigment epithelium (RPE). TIMP3 gene is mapped to human chromosome 22q12.3 and is a CLOCK-dependent diurnal gene. TIMP proteins display a three-lobed structure and have conserved cysteine residues.
Acciones bioquímicas o fisiológicas
The TIMPs are endogenous inhibitors of the matrix metalloproteinases (MMPs). TIMP-3 inhibits ADAM-17 (TACE) at nanomolar concentrations and also inhibits other metalloproteinases (sheddases) that mediate the shedding of soluble receptors and other proteins from the surface of cells.
Tissue inhibitor of metalloproteinases 3 (TIMP3) is a matrix metalloproteinase inhibitor. TIMP proteins comprise the N-terminal region, which aids in the matrix metalloproteinase interaction. The C-terminal region is crucial for extracellular matrix (ECM) interaction. Elevated expression of TIMP3 favors apoptosis in cancer types. Its interaction with the vascular endothelial growth factor (VEGFR-2) leads to the regulation of angiogenesis. TIMP3 also aids in protection against ultra-violet (UV)-induced cellular responses. Missense mutations in the TIMP3 gene are implicated in Sorsby′s fundus dystrophy (SFD). Mutations in the TIMP3 gene also leads to increased accumulation of the protein resulting in the thickening of the Bruch membrane. This, in turn, reduces membrane permeability towards nutrients and metabolites.
Forma física
Lyophilized from a 0.2 μm filtered solution in 25 mM Tris and 0.15 M sodium chloride, pH 7.5.
Nota de análisis
The biological activity is measured by its ability to inhibit human MMP-2 hydrolysis of a peptide substrate.
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 1
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Ruth Appeltant et al.
Animal science journal = Nihon chikusan Gakkaiho, 88(9), 1279-1290 (2017-01-27)
In vitro maturation (IVM) in serum causes hampered expansion of porcine cumulus-oocyte complexes (COCs) due to excessive alpha
Daniel Ardeljan et al.
European journal of human genetics : EJHG, 21(10), 1152-1157 (2013-02-21)
Age-related macular degeneration (AMD) is a leading cause of irreversible central visual loss in the elderly. A recent genome-wide association studies (GWAS) reported that rs9621532 near the tissue inhibitor of metalloproteinase 3 (TIMP3)/synapsin III (SYN3) region of 22q12.3 is associated
Sunyoung Park et al.
International journal of molecular sciences, 20(4) (2019-02-20)
The human skin is the outermost physical barrier and has its own circadian machinery that works either cooperatively with the central clock, or autonomously. Circadian rhythms have been observed in many functions related to epidermal homeostasis including hydration and inflammation
S M Silbiger et al.
Gene, 141(2), 293-297 (1994-04-20)
Proteins of the tissue inhibitor of metalloproteinase (TIMP) family bind and inactivate matrix metalloproteinases such as collagenases and gelatinases. We report the cloning and sequencing of cDNAs encoding a novel human TIMP, which we designated TIMP-3, the third member of
K J Leco et al.
The Journal of biological chemistry, 269(12), 9352-9360 (1994-03-25)
We have isolated cDNA clones corresponding to a novel mouse metalloproteinase inhibitor. Five overlapping cDNA clones contain most of the information for a prominent 4.5-kilobase transcript that was detected in RNA from mouse fibroblasts and adult tissues. Sequence analysis revealed
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico