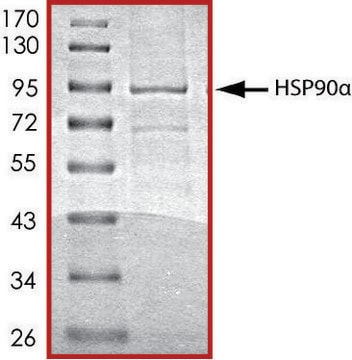
SRP0182
Hsp90a Active human
recombinant, expressed in E. coli, ≥80% (SDS-PAGE)
Sinónimos:
HSP86, HSPCAL3, Heat shock protein 90 kDa α, LAP2, NY-REN-38 (renal carcinoma antigen)
About This Item
Productos recomendados
biological source
human
recombinant
expressed in E. coli
assay
≥80% (SDS-PAGE)
form
aqueous solution
potency
≥8 nM
mol wt
85.5 kDa
packaging
pkg of 200 μg
storage condition
avoid repeated freeze/thaw cycles
concentration
>0.02 mg/mL
NCBI accession no.
UniProt accession no.
shipped in
dry ice
storage temp.
−70°C
Gene Information
human ... HSP90AA2(3324)
General description
Application
Biochem/physiol Actions
Physical form
Preparation Note
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Repr. 1B - Skin Irrit. 2
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 1
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico