SAE0045
HRV-3C Protease.
N-Terminal His tagged recombinant protein, aqueous solution, 0.8-1.2 mg/mL
Sinónimos:
Human Rhinovirus 3C Protease, Levlfqgp site protease, PreScission Protease
About This Item
Productos recomendados
biological source
human (human Rhinovirus Type 14)
Quality Level
assay
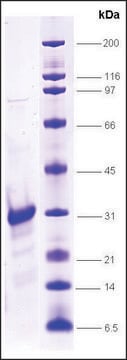
≥90% (SDS-PAGE)
form
aqueous solution
specific activity
≥5000 U/mg
mol wt
21 kDa
concentration
0.8-1.2 mg/mL
technique(s)
protein purification: suitable
suitability
suitable for protein modification
application(s)
life science and biopharma
shipped in
dry ice
storage temp.
−20°C
General description
Proteolytic cleavage occurs between the Gln and Gly residues.
HRV-3C protease is a useful tool to cleave recombinant proteins that are expressed as a fusion protein with this sequence, between the carrier domain and the protein of interest.
This recombinant version contains a six-histidine tag and can be easily removed by IMAC chromatography.
Application
Unit Definition
Physical form
Storage Class
10 - Combustible liquids
wgk_germany
WGK 2
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Proteases for biotinylated tag removal for protein purification workflows with related reagents and technical resources.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico