R7257
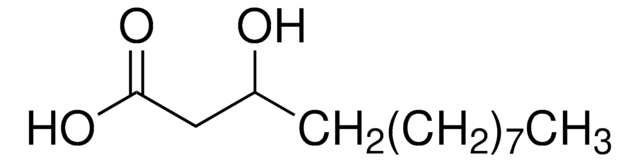
Ricinoleic acid
≥95%
Sinónimos:
Ricinelaidic acid, (R)-12-Hydroxy-cis-9-octadecenoic acid, 12-Hydroxyoleic acid
About This Item
Productos recomendados
biological source
natural (organic)
assay
≥95%
form
liquid
density
0.940 g/mL at 20 °C (lit.)
functional group
carboxylic acid
hydroxyl
lipid type
unsaturated FAs
shipped in
ambient
storage temp.
−20°C
SMILES string
CCCCCC[C@@H](O)C\C=C/CCCCCCCC(O)=O
InChI
1S/C18H34O3/c1-2-3-4-11-14-17(19)15-12-9-7-5-6-8-10-13-16-18(20)21/h9,12,17,19H,2-8,10-11,13-16H2,1H3,(H,20,21)/b12-9-/t17-/m1/s1
InChI key
WBHHMMIMDMUBKC-QJWNTBNXSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- Multiplexed PLGA scaffolds with nitric oxide-releasing zinc oxide and melatonin-modulated extracellular vesicles for severe chronic kidney disease.: This innovative study utilizes ricinoleic acid in the fabrication of multiplexed PLGA scaffolds, demonstrating its effectiveness in enhancing the therapeutic efficacy of treatments for chronic kidney disease. The research underscores the potential of ricinoleic acid in medical applications, particularly in regenerative medicine and drug delivery systems (Rhim WK et al., 2024).
- Optimizing the enzymatic production of biolubricants by the Taguchi method: Esterification of the free fatty acids from castor oil with 2-ethyl-1-hexanol catalyzed by Eversa Transform 2.0.: Demonstrates the industrial application of ricinoleic acid in producing biolubricants, emphasizing its economic and environmental benefits. The study provides insights into the sustainable production processes and industrial applications of ricinoleic acid (Monteiro RRC et al., 2024).
Packaging
Storage Class
10 - Combustible liquids
wgk_germany
WGK 3
flash_point_f
435.2 °F - closed cup
flash_point_c
224 °C - closed cup
ppe
Eyeshields, Gloves, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico