M7571
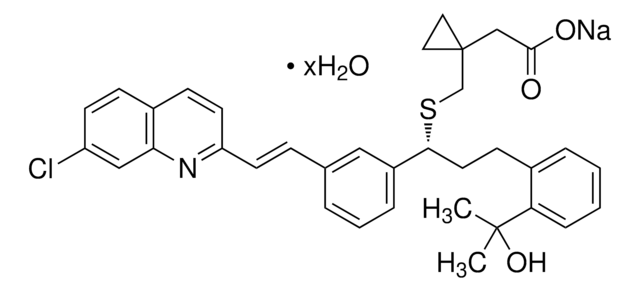
MK-571 sodium salt hydrate
≥95% (HPLC), powder, leukotriene D4 antagonist
Sinónimos:
5-(3-(2-(7-Chloroquinolin-2-yl)ethenyl)phenyl)-8-dimethylcarbamyl-4,6-dithiaoctanoic acid sodium salt hydrate, L-660711
About This Item
Productos recomendados
Nombre del producto
MK-571 sodium salt hydrate, ≥95% (HPLC)
Nivel de calidad
Ensayo
≥95% (HPLC)
Formulario
powder
condiciones de almacenamiento
desiccated
color
white to beige
solubilidad
H2O: 15 mg/mL, clear
emisor
Merck & Co., Inc., Kenilworth, NJ, U.S.
Condiciones de envío
wet ice
temp. de almacenamiento
−20°C
cadena SMILES
O.[Na+].CN(C)C(=O)CCSC(SCCC([O-])=O)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1
InChI
1S/C26H27ClN2O3S2.Na.H2O/c1-29(2)24(30)12-14-33-26(34-15-13-25(31)32)20-5-3-4-18(16-20)6-10-22-11-8-19-7-9-21(27)17-23(19)28-22;;/h3-11,16-17,26H,12-15H2,1-2H3,(H,31,32);;1H2/q;+1;/p-1/b10-6+;;
Clave InChI
MSHRPLRGSQECLY-DOLBFOAYSA-M
Aplicación
- as an efflux inhibitor for monitoring multidrug resistance protein (MRP)-function and to avoid redundancy of other transporters
- to assess its effect on cell proliferation and 2D-migration in vitro in various cell lines of glioblastoma multiforme (GBM)
- as multidrug resistance (MDR) transporter inhibitor to study its effects in ovarian cancer cells
- as specific inhibitors of ABCC1/2 to investigate transport, toxicity, flow cytometry and arsenic efflux
Acciones bioquímicas o fisiológicas
Características y beneficios
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We offer many products related to leukotriene receptors for your research needs.
We offer many products related to leukotriene receptors for your research needs.
We offer many products related to leukotriene receptors for your research needs.
We offer many products related to leukotriene receptors for your research needs.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico