C6506
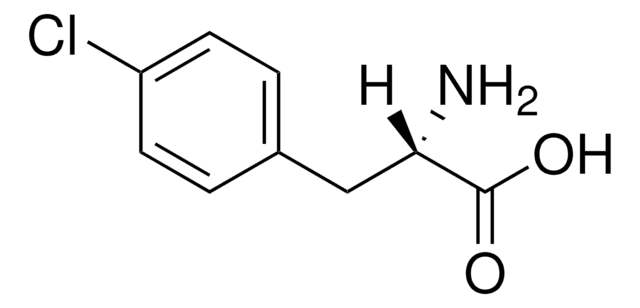
4-Chloro-DL-phenylalanine
Sinónimos:
PCP, PCPA
About This Item
Productos recomendados
form
solid
greener alternative product score
old score: 2
new score: 1
Find out more about DOZN™ Scoring
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Designing Safer Chemicals
Design for Energy Efficiency
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
mp
>240 °C (dec.) (lit.)
greener alternative category
storage temp.
room temp
SMILES string
NC(Cc1ccc(Cl)cc1)C(O)=O
InChI
1S/C9H10ClNO2/c10-7-3-1-6(2-4-7)5-8(11)9(12)13/h1-4,8H,5,11H2,(H,12,13)
InChI key
NIGWMJHCCYYCSF-UHFFFAOYSA-N
Gene Information
human ... TPH1(7166) , TPH2(121278)
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- as tryptophan hydroxylase 1 (TPH1) inhibitor to treat kras+ male zebrafish
- to induce insomnia in rat models
- used to treat embryos to examine its effect on serotonin
- for the selection of Enterococcus faecalis transformants with pESentA32 plasmid
- to feed flies to explore serotonin effect
Biochem/physiol Actions
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Skin Sens. 1
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Chromatograms
application for HPLCNuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico