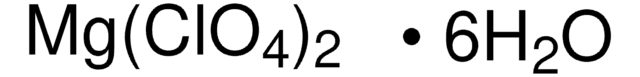222283
Magnesium perchlorate
ACS reagent
About This Item
Productos recomendados
grade
ACS reagent
Quality Level
form
flakes
powder, chunks or granules
reaction suitability
reagent type: oxidant
concentration
>10% Mg (EDTA titration)
impurities
≤0.005 meq/g Titr. free acid
≤0.025 meq/g Titr. base
loss
≤8% loss on drying
suitability
passes test for moisture absorption
SMILES string
[Mg++].[O-]Cl(=O)(=O)=O.[O-]Cl(=O)(=O)=O
InChI
1S/2ClHO4.Mg/c2*2-1(3,4)5;/h2*(H,2,3,4,5);/q;;+2/p-2
InChI key
MPCRDALPQLDDFX-UHFFFAOYSA-L
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Preparation of α-aminophosphonates.
- Enantioselective Diels-Alder reaction between cyclopentadiene and 3-acryloyl-1,3-oxazolin-2-one.
- Preparation of imines and phenylhydrazones.
- Protection of alcohols in the form of t-butyl ethers.
- α-Aminophosphonates via three-component reaction between an amine, an aldehyde or a ketone and a di-/trialkyl phosphite.
- Imines and phenylhydrazones by the condensation of carbonyl compounds with amines and phenylhydrazine.
- Knoevenagel adducts via Knoevenagel condensation between β-diketones and aliphatic or aromatic aldehydes.
signalword
Danger
hcodes
Hazard Classifications
Eye Irrit. 2 - Ox. Sol. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
5.1A - Strongly oxidizing hazardous materials
wgk_germany
WGK 1
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico