15450
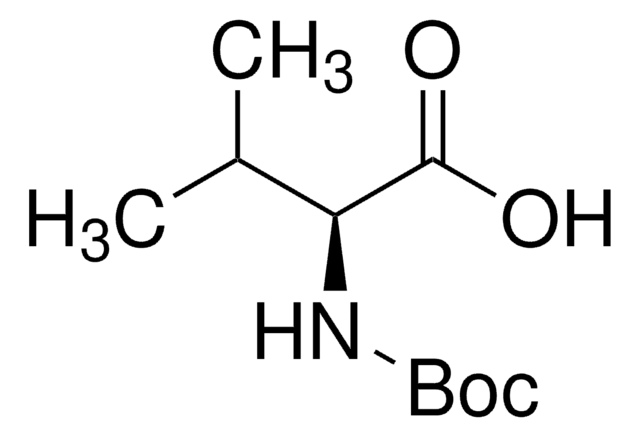
Boc-Leu-OH hydrate
≥99.0% (HPLC)
Sinónimos:
Boc-L-leucine
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
(CH3)2CHCH2CH(COOH)NHCOOC(CH3)3 xH2O
Peso molecular:
231.29 (anhydrous basis)
EC Number:
MDL number:
UNSPSC Code:
12352209
eCl@ss:
32160406
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
≥99.0% (HPLC)
form
solid
optical activity
[α]20/D −25±0.5°, c = 2% in acetic acid
reaction suitability
reaction type: Boc solid-phase peptide synthesis
reaction type: C-H Activation
reagent type: ligand
reaction type: Peptide Synthesis
mp
85-90 °C
application(s)
peptide synthesis
functional group
amine
carboxylic acid
SMILES string
CC(C)C[C@H](NC(OC(C)(C)C)=O)C(O)=O
Application
Boc-Leu-OH (Boc-L-leucine) was used in the synthesis of a potent cytotoxin, PM-94128.
Boc-protected leucine (Boc-Leu-OH) can be used to generate combinatorial peptide libraries and also to synthesize peptide models to study structure-activity relationships.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Multicyclic polypeptide model compounds. 1. Synthesis of a tricyclic amphiphilic. alpha.-helical peptide using an oxime resin, segment-condensation approach.
Osapay G, et al.
Journal of the American Chemical Society, 112(16), 6046-6051 (1990)
Masaru Enomoto et al.
The Journal of organic chemistry, 74(19), 7566-7569 (2009-09-03)
The enantioselective total synthesis of PM-94128, a potent cytotoxin of microbial origin, was accomplished by a concise nine-step sequence of reactions in 14% overall yield from N-Boc-l-leucine. The synthesis of Y-05460M-A, a one-carbon lower homologue of PM-94128, was also achieved
Screening of mixture combinatorial libraries for chiral selectors: a reciprocal chromatographic approach using enantiomeric libraries.
Wu Y, et al.
Analytical Chemistry, 71(9), 1688-1691 (1999)
The synthesis and screening of a combinatorial peptide library for affinity ligands for glycosylated haemoglobin1.
Chen B, et al.
Biosensors And Bioelectronics, 13(7-8), 779-785 (1998)
Hyun-Woong Cho et al.
PloS one, 14(6), e0217745-e0217745 (2019-06-21)
The aim of this study was to investigate the short-term efficacy and safety of Poly-gamma-glutamic acid (γ-PGA) and the immunologic changes in patients with CIN 1. Participants were randomly assigned to one of two groups and orally treated with placebo
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico