8.52107
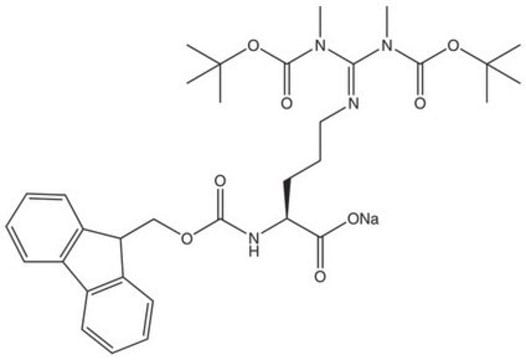
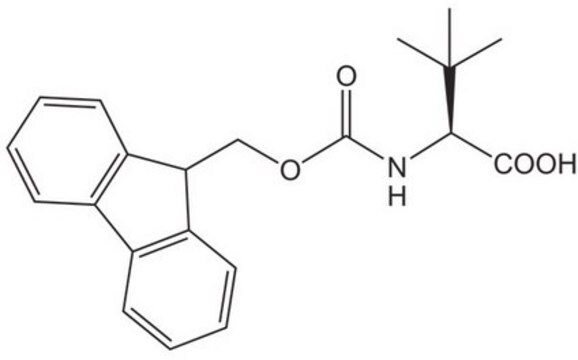
Fmoc-ADMA(Pbf)-OH
≥98% (TLC), for peptide synthesis, Novabiochem®
Sinónimos:
Fmoc-ADMA(Pbf)-OH, N-α-Fmoc-N,N-ω-dimethyl-N-ωÆ-(2,2,4,6,7-pentamethyldihydrobenzofuran-5-sulfonyl)-L-arginine
About This Item
Productos recomendados
product name
Fmoc-ADMA(Pbf)-OH, Novabiochem®
Quality Level
product line
Novabiochem®
assay
≥96.0% (HPLC)
≥98% (TLC)
form
powder
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
15-25°C
General description
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references
[1] S. Rothbart, et al. (2012) Methods Enzymol., 512, 107.
Application
- Effect of arginine methylation on the RNA recognition and cellular uptake of Tat-derived peptides: Studies the use of Fmoc-ADMA(Pbf)-OH in modifying peptides to enhance their cellular uptake, relevant for drug delivery systems and therapeutic molecule development (JH Li et al., 2015).
- Advances in Fmoc solid-phase peptide synthesis: Reviews the role of Fmoc-ADMA(Pbf)-OH in advancing peptide synthesis techniques, impacting the field of material science through the development of novel synthetic strategies and applications in biomaterials (R Behrendt, J Offer, 2016).
Linkage
Analysis Note
Appearance of substance (visual): powder
Identity (IR): passes test
Purity (TLC(157A)): ≥ 98 %
Enantiomeric purity: ≥ 99.0 % (a/a)
Assay (HPLC, area%): ≥ 96.0 % (a/a)
Solubility (1 mmole in 2 ml DMF): clearly soluble
Water (K. F.): ≤ 2.0 %
To see the solvent systems used for TLC of Novabiochem® products please click here.
Legal Information
Storage Class
11 - Combustible Solids
wgk_germany
WGK 2
flash_point_f
Not applicable
flash_point_c
Not applicable
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
Unnatural amino acids, the non-proteinogenic amino acids that either occur naturally or are chemically synthesized, are becoming more and more important as tools for modern drug discovery research.
Unnatural amino acids, the non-proteinogenic amino acids that either occur naturally or are chemically synthesized, are becoming more and more important as tools for modern drug discovery research.
Unnatural amino acids, the non-proteinogenic amino acids that either occur naturally or are chemically synthesized, are becoming more and more important as tools for modern drug discovery research.
Unnatural amino acids, the non-proteinogenic amino acids that either occur naturally or are chemically synthesized, are becoming more and more important as tools for modern drug discovery research.
Protocolos
We provide an overview of our available reagents, together with recommendations and details of their use for synthesis of peptides containing post-translationally modified amino acids.
We provide an overview of our available reagents, together with recommendations and details of their use for synthesis of peptides containing post-translationally modified amino acids.
We provide an overview of our available reagents, together with recommendations and details of their use for synthesis of peptides containing post-translationally modified amino acids.
We provide an overview of our available reagents, together with recommendations and details of their use for synthesis of peptides containing post-translationally modified amino acids.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico