930571
Pomalidomide-piperidine-carboxylic acid
≥95%
Sinónimos:
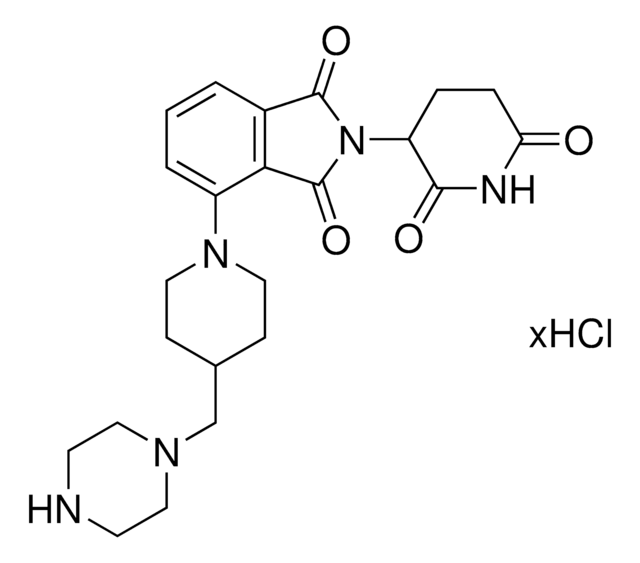
1-[2-(2,6-Dioxo-3-piperidinyl)-2,3-dihydro-1,3-dioxo-1H-isoindol-5-yl], 1-[2-(2,6-Dioxo-3-piperidyl)-1,3-dioxo-isoindolin-5-yl]piperidine-4-carboxylic acid, 4-Piperidinecarboxylic acid
About This Item
Productos recomendados
ligand
pomalidomide
Quality Level
assay
≥95%
form
powder
functional group
carboxylic acid
storage temp.
2-8°C
SMILES string
O=C(O)C1CCN(C2=CC=C3C(=O)N(C(=O)C3=C2)C4C(=O)NC(=O)CC4)CC1
Categorías relacionadas
Application
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Other Notes
Destruction of DNA-Binding Proteins by Programmable Oligonucleotide PROTAC (O′PROTAC): Effective Targeting of LEF1 and ERG
Small-Molecule PROTACS: New Approaches to Protein Degradation
Targeted Protein Degradation: from Chemical Biology to Drug Discovery
Impact of linker length on the activity of PROTACs
signalword
Danger
hcodes
Hazard Classifications
Repr. 1B
Storage Class
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
wgk_germany
WGK 3
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico