855286
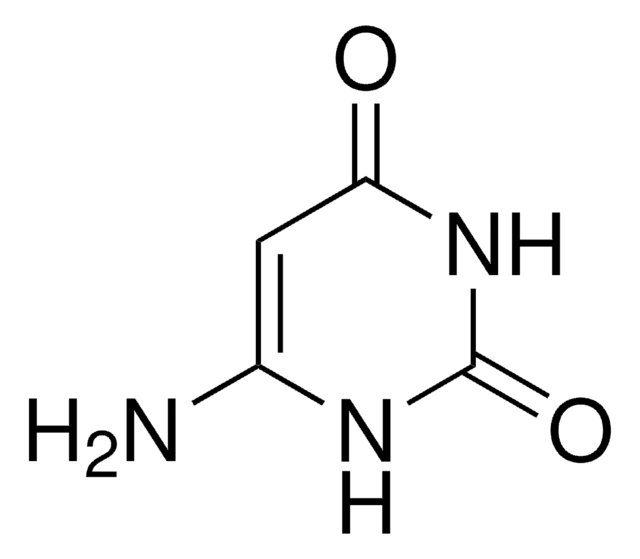
5-Aminouracil
98%
Sinónimos:
5-Amino-2,4-dihydroxypyrimidine, 5-Amino-2,4-pyrimidinediol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
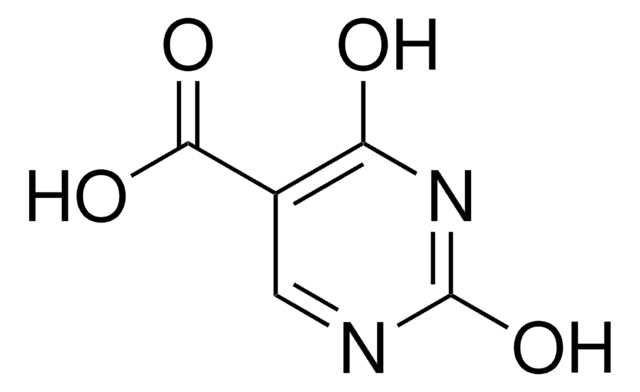
C4H5N3O2
Número de CAS:
Peso molecular:
127.10
Beilstein/REAXYS Number:
127250
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
Quality Level
assay
98%
form
powder
mp
>300 °C (lit.)
SMILES string
NC1=CNC(=O)NC1=O
InChI
1S/C4H5N3O2/c5-2-1-6-4(9)7-3(2)8/h1H,5H2,(H2,6,7,8,9)
InChI key
BISHACNKZIBDFM-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Paulina Spisz et al.
International journal of molecular sciences, 21(17) (2020-09-05)
Hypoxia-a hallmark of solid tumors-dramatically impairs radiotherapy, one of the most common anticancer modalities. The adverse effect of the low-oxygen state can be eliminated by the concomitant use of a hypoxic cell radiosensitizer. In the present paper, we show that
Asmaa M Fahim et al.
Current computer-aided drug design, 16(4), 486-499 (2019-07-11)
In this investigation, 2-cyano-N-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl) acetamide (3) reacts with dimethylformamide dimethyl acetal (DMF-DMA) to afford the corresponding (E)- 2-cyano-3-(dimethylamino)-N-(2,4-dioxo-1,2,3,4-tetrahydropyrimidin-5-yl)acrylam-ide (4) utilizing microwave irradiation. The condensation reactions of acrylamide derivative 4 with hydrazine derivatives obtain pyrazole derivatives 6a and 6b; respectively. The
F Cortés et al.
Experimental cell research, 148(2), 503-507 (1983-10-15)
Meristematic cells of Allium cepa were treated with 5-amino-uracil (5-AU) while incorporating 5-bromodeoxyuridine (BrdU) into DNA until complete inhibition of mitosis was obtained. The pattern of BrdU substitution in interphase nuclei detected by FPG technique in the cells so treated
A González-Fernández et al.
Mutation research, 149(2), 275-281 (1985-04-01)
Proliferating plant cells treated during the late S period with 5-aminouracil (AU), give the typical response that DNA-damaging agents induce, characterized by: an important mitotic delay, and a potentiation of the chromosome damage by caffeine post-treatment. The study of labelled
J E Thomas et al.
Cell and tissue kinetics, 16(3), 285-301 (1983-05-01)
The influence of 5-amino uracil (5-AU) was investigated on the cell cycle of log growth and division-synchronized Tetrahymena pyriformis GL. The division index of log growth phase Tetrahymena was suppressed by 50% after 40 min in 8 mM 5-AU. Cells
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico