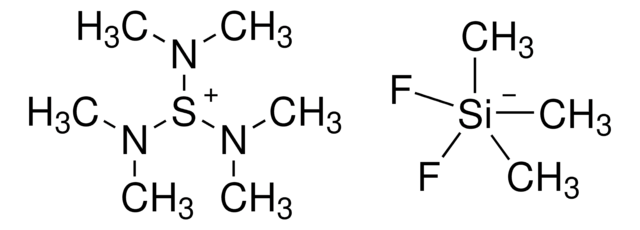
330744
Tetrapropylammonium perruthenate
97%
Sinónimos:
TPAP
About This Item
Productos recomendados
assay
97%
form
solid
reaction suitability
reagent type: oxidant
mp
~160 °C (dec.) (lit.)
SMILES string
[O-][Ru](=O)(=O)=O.CCC[N+](CCC)(CCC)CCC
InChI
1S/C12H28N.4O.Ru/c1-5-9-13(10-6-2,11-7-3)12-8-4;;;;;/h5-12H2,1-4H3;;;;;/q+1;;;;-1;
InChI key
NQSIKKSFBQCBSI-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- For the conversion of sulfides to sulfones by oxidation reaction.
- In the isomerization of allylic alcohols into the corresponding saturated carbonyl derivatives.
- Along with N-methylmorpholine N-oxide (NMO) for the cleavage of glycol to carboxylic acids.
TPAP can also be used as an oxidizing reagent:
- For the oxidation of benzyl alcohol to benzaldehyde and steroidal alcohols to corresponding ketones.
- To convert N,N′-dihydroxyimidazolidines to nitronyl nitroxide free radicals.
- To oxidize hydroxyl-substituted tri-n-butylammonium trifluoroborates to aldehydes and ketones without concomitant cleavage of the carbon-boron bond.
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis, and are some of the organic chemist’s most powerful tools for creating novel products. Below is a list of the most commonly used oxidizing and reducing agents currently available in our catalog.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico