126462
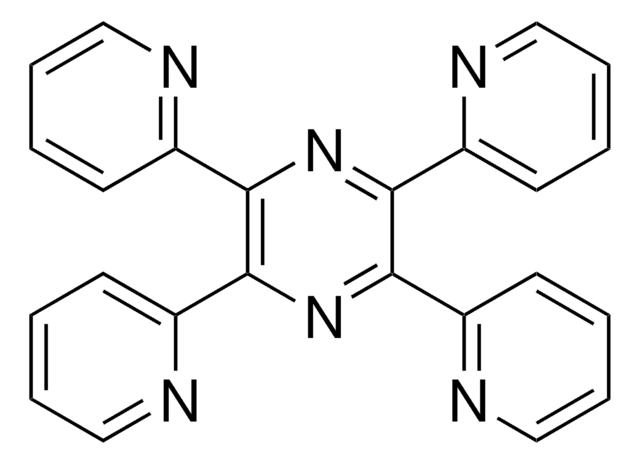
6,7-Dimethyl-2,3-di(2-pyridyl)quinoxaline
98%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula empírica (notación de Hill):
C20H16N4
Número de CAS:
Peso molecular:
312.37
Número CE:
Número MDL:
Código UNSPSC:
12352100
ID de la sustancia en PubChem:
NACRES:
NA.22
Productos recomendados
Ensayo
98%
Formulario
powder
mp
191-193 °C (lit.)
cadena SMILES
Cc1cc2nc(-c3ccccn3)c(nc2cc1C)-c4ccccn4
InChI
1S/C20H16N4/c1-13-11-17-18(12-14(13)2)24-20(16-8-4-6-10-22-16)19(23-17)15-7-3-5-9-21-15/h3-12H,1-2H3
Clave InChI
NACXMBPTPBZQHY-UHFFFAOYSA-N
Categorías relacionadas
Aplicación
6,7-Dimethyl-2,3-di-(2-pyridyl)quinoxaline has been used as an internal standard to investigate the clinical pharmacokinetics of nelfinavir mesylate, a potent inhibitor of HIV-1 protease.
Palabra de señalización
Warning
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Órganos de actuación
Respiratory system
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
B Louveau et al.
Biomedical chromatography : BMC, 30(12), 2009-2015 (2016-06-10)
A precise and accurate high-performance liquid chromatography (HPLC) quantification method of rifampicin in human plasma was developed and validated using ultraviolet detection after an automatized solid-phase extraction. The method was validated with respect to selectivity, extraction recovery, linearity, intra- and
Salmaan Kanji et al.
Clinical pharmacokinetics, 59(3), 327-334 (2019-09-01)
Sustained low-efficiency dialysis (SLED) is a hybrid form of dialysis that is increasingly used in critically ill patients with kidney injury and hemodynamic instability. Antimicrobial dosing for patients receiving SLED is informed by pharmacokinetic studies that describe the drug clearance.
E Y Wu et al.
Journal of chromatography. B, Biomedical sciences and applications, 695(2), 373-380 (1997-08-01)
Nelfinavir mesylate, a potent and orally bioavailable inhibitor of HIV-1 protease (Ki=2 nM), has undergone Phase III clinical evaluation in a large population of HIV-positive patients. A high-performance liquid chromatography analytical method was developed to determine the pharmacokinetic parameters of
Valeria Avataneo et al.
The Journal of antimicrobial chemotherapy, 75(7), 1772-1777 (2020-05-04)
Remdesivir has received significant attention for its potential application in the treatment of COVID-19, caused by SARS-CoV-2. Remdesivir has already been tested for Ebola virus disease treatment and found to have activity against SARS and MERS coronaviruses. The remdesivir core
Sara Baldelli et al.
Therapeutic drug monitoring, 36(6), 739-745 (2014-04-18)
Recently, the European Medicines Agency (EMA) has released new guidelines on the validation of bioanalytical methods. In this work, we compared the analytical performance of 2 high-performance liquid chromatography with tandem mass spectrometry methods designed for the quantification of the
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico