125318
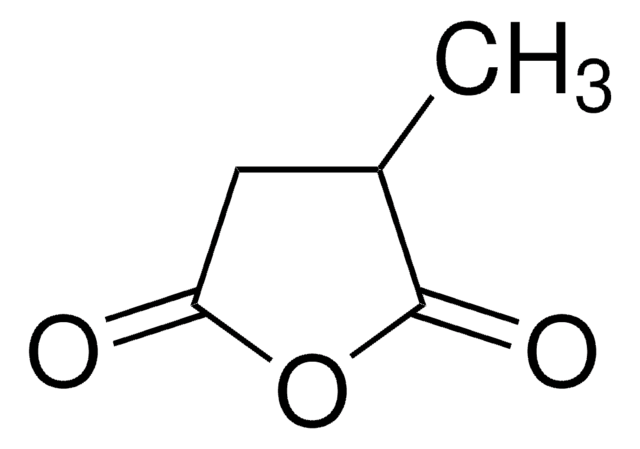
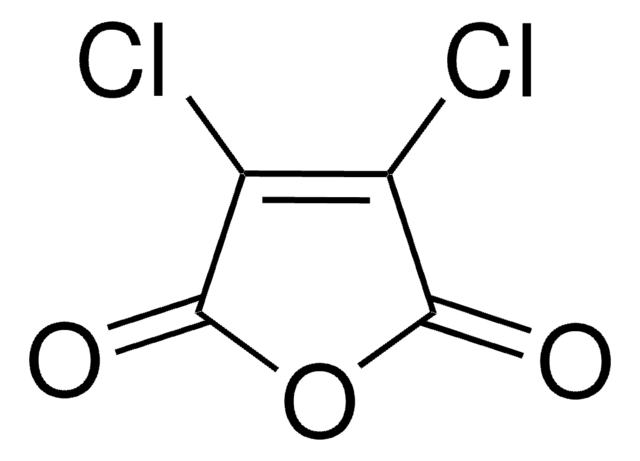
Citraconic anhydride
98%
Sinónimos:
2-Methylmaleic anhydride, 3-Methyl-2,5-furandione, Citraconic acid anhydride, Methylmaleic anhydride, Monomethylmaleic anhydride
About This Item
Productos recomendados
densidad de vapor
4 (vs air)
Nivel de calidad
Ensayo
98%
Formulario
liquid
índice de refracción
n20/D 1.471 (lit.)
bp
213-214 °C (lit.)
mp
6-10 °C (lit.)
densidad
1.247 g/mL at 25 °C (lit.)
cadena SMILES
CC1=CC(=O)OC1=O
InChI
1S/C5H4O3/c1-3-2-4(6)8-5(3)7/h2H,1H3
Clave InChI
AYKYXWQEBUNJCN-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- As an electrolyte additive for high-temperature pouch lithium-ion batteries. Citraconic anhydride reduces the interfacial impedance of pouch cells during high-temperature storage and enhances their stability.
- As a pH-sensitive linker to surface functionalization of biomolecules used in drug delivery systems. The high pH sensitivity of citraconic anhydride conjugates is attributed to the presence of a double bond that restricts the separation between the amide and carboxylic acid groups.
- As a reagent to synthesize new thiopyrano[2,3-d][1,3]thiazole derivatives via hetero-Diels–Alder reactions. These thiopyrano derivatives exhibit diverse biological activities such as anticancer, antiviral, and antitrypanosomal.
- As a co-monomer in the ring-opening polymerization with d-xylose 3,5-anhydrosugar derivative to form novel sugar-derived polyesters, with up to 100% renewable content. This can serve as a sustainable feedstock for polymer synthesis.
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Eye Dam. 1 - Resp. Sens. 1 - Skin Corr. 1B - Skin Sens. 1
Código de clase de almacenamiento
8A - Combustible corrosive hazardous materials
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
231.8 °F - closed cup
Punto de inflamabilidad (°C)
111 °C - closed cup
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico