CC1890
Aggrecan Interglobular Domain, Recombinant, His-tagged
Synonym(s):
Aggrecan-IGD1
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
UNSPSC Code:
12352204
eCl@ss:
32160405
NACRES:
NA.77
Recommended Products
biological source
human
Quality Level
form
liquid
manufacturer/tradename
Chemicon®
NCBI accession no.
UniProt accession no.
shipped in
dry ice
Gene Information
human ... ACAN(176)
General description
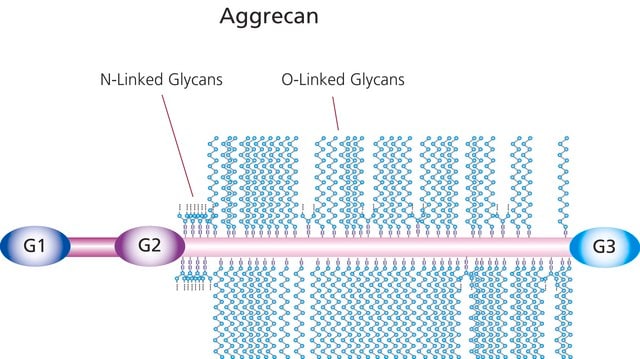
Aggrecan is a large aggregating proteoglycan of articular cartilage. It is also found in aorta, discs and tendons [Knudsen & Knudsen, 2001; Watanabe et al., 1998]. The aggrecan core protein consists of 2317 amino acids [Watanabe et al., 1998]. Up to 130 glucosaminoglycan chains are attached to the core protein and the total molecular mass can reach 2.2 - 3.0 x 106 Daltons [Hardingham & Fosang, 1992]. Within the aggrecan molecule 3 global domains G1, G2 and G3 can be distinguished. Domains G1 and G2 are connected by a rod-shaped polypeptide called interglobular domain (IGD), while the sequence between domains G2 and G3 contains attachment regions for keratan sulfate and chondroitin sulfate chains. Aggrecan interacts via the G1domain with hyaluronan and link protein to form large aggregates. Such aggregates can contain up to 50-100 aggrecan monomers noncovalently bound to a single hyaluronan chain through 2 link proteins [Knudsen & Knudsen, 2001; Watanabe et al., 1998; Hardingham & Fosang, 1992]. The aggregates form a hydrated gel-like structure, which endowes cartilage with resistibility to compression and deformation. Degradation of aggrecan appears to initiate at the C-terminus. The population of aggrecan molecules without the G3 domain increases with aging [Dudhia et al., 1996]. Isolated aggrecanases cleave aggrecan at 4 sites within the chrondroitin sulfate-rich region (sites E1667 -G1668, E1480-G1481, E1771 - A1772, E1871 - L1872) and 1 site within the interglobular domain (E373 - A374) [Tortorella et al., 2000]. Cleavage at the latter site had been documented by analysis of cartilage proteoglycan breakdown products in rheumatoid and osteoarthritis [Lohmander et al., 1993]. To measure aggrecanase activity, an artificial recombinant protein composed of aggrecan interglobular domain with flanking FLAG-sequence and human immunoglobulin G1 constant region was first used by Hughes et al. [Hughes et al., 1997].
Molecular form: The polypeptide connecting human aggrecan globular domains 1 and 2 (T331 - G458) is expressed in E. coli with a C-terminal twin His-tag {6His-L-E-6His}. The recombinant protein contains cleavage sites for aggrecanases (E373 - A374) and matrix metalloproteinases (N341 - F342). It comprises the following amino acids: T A E D F V D I P E N<> F F G V G G E E D I T V Q T V T W P D M E L P L P R N I T E G E <>A R G S V I L T V K P I F E V S P S P L E P E E P F T F A P E I G A T A F A E V E N E T G E A T R P W G F P T P G L G P A T A F T S E D L V V Q V T A V P G Q P H L P G G (His-tag)
Main cleavage sites are indicated by <> inclusions.. The calculated Mr of the His-tagged protein is 15,493 Da.
Purity: The recombinant aggrecan interglobular domain appears as a major band at about 21 kDa in SDS-PAGE. It represents more than 90% of total protein in the preparation.
Applications: Aggrecan interglobular domain is used as substrate for aggrecanases and matrix metalloproteinases. For proteinase activity measurements, the protein is incubated with proteinase for various time intervals. Thereafter, aliquots of the incubation mixture are analyzed by SDS-PAGE or by ELISA.Upon cleavage with aggrecanases the apparent Mr of aggrecan interlobular domain in SDS-PAGE is reduced from 21 kDa to about 13 kDa. Quantitative measurement of aggrecanase cleavage requires a neoepitop antibody with specificity for the N-terminus A R G S V I L T . . . appearing upon hydrolysis. The fragment with the newly formed N-terminus is fixed by the neoepitop antibody to a microplate and quantified with an anti-His-tag antibody. In analogy, cleavage by matrix metalloproteinases can be measured with antibodies to neoepitopes appearing upon action of these enzymes.
Main cleavage sites are indicated by <> inclusions.. The calculated Mr of the His-tagged protein is 15,493 Da.
Purity: The recombinant aggrecan interglobular domain appears as a major band at about 21 kDa in SDS-PAGE. It represents more than 90% of total protein in the preparation.
Applications: Aggrecan interglobular domain is used as substrate for aggrecanases and matrix metalloproteinases. For proteinase activity measurements, the protein is incubated with proteinase for various time intervals. Thereafter, aliquots of the incubation mixture are analyzed by SDS-PAGE or by ELISA.Upon cleavage with aggrecanases the apparent Mr of aggrecan interlobular domain in SDS-PAGE is reduced from 21 kDa to about 13 kDa. Quantitative measurement of aggrecanase cleavage requires a neoepitop antibody with specificity for the N-terminus A R G S V I L T . . . appearing upon hydrolysis. The fragment with the newly formed N-terminus is fixed by the neoepitop antibody to a microplate and quantified with an anti-His-tag antibody. In analogy, cleavage by matrix metalloproteinases can be measured with antibodies to neoepitopes appearing upon action of these enzymes.
Product Source: Human
Physical form
The calculated Mr of the His-tagged protein is 15,493 Da. The protein is solubilized in 50 mM Tris-HCI, pH 7.5, 150 mM NaCl, 5 mM CaCl2.
Storage and Stability
MT5-MMP is stable until the expiry date given on the label of stored at -70°C. The protein can be kept at -20°C for several weeks and on ice for several days. Repeated freezing and thawing should be avoided.
Legal Information
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
Disclaimer
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C B Knudson et al.
Seminars in cell & developmental biology, 12(2), 69-78 (2001-04-09)
The predominant proteoglycan present in cartilage is the large chondroitin sulfate proteoglycan 'aggrecan'. Following its secretion, aggrecan self-assembles into a supramolecular structure with as many as 50 monomers bound to a filament of hyaluronan. Aggrecan serves a direct, primary role
E U Sumer et al.
Osteoarthritis and cartilage, 15(2), 212-221 (2006-09-26)
Aggrecan is the major proteoglycan in articular cartilage and is known to be degraded by various proteases, including matrix metalloproteinases (MMPs). The present study was undertaken to develop immunoassays detecting aggrecan and its fragments generated by MMP and non-MMP-mediated proteolysis.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service