P14858
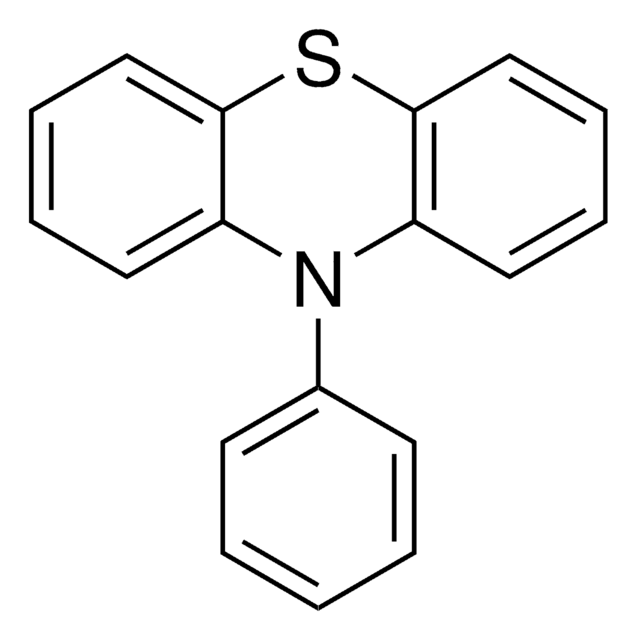
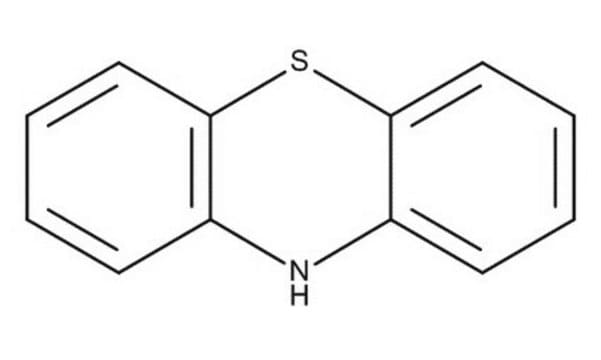
Phenoxazine
97%
Synonym(s):
5,6-Dibenzo-1,4-oxazine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C12H9NO
CAS Number:
Molecular Weight:
183.21
Beilstein:
143234
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
powder
mp
156-159 °C (lit.)
SMILES string
N1c2ccccc2Oc3ccccc13
InChI
1S/C12H9NO/c1-3-7-11-9(5-1)13-10-6-2-4-8-12(10)14-11/h1-8,13H
InChI key
TZMSYXZUNZXBOL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Steve H Thorne et al.
Molecular cancer therapeutics, 8(2), 333-341 (2009-02-05)
We report the discovery of a new prodrug, 6-chloro-9-nitro-5-oxo-5H-benzo(a)phenoxazine (CNOB). This prodrug is efficiently activated by ChrR6, the highly active prodrug activating bacterial enzyme we have previously developed. The CNOB/ChrR6 therapy was effective in killing several cancer cell lines in
Kyoko Hayashi et al.
Journal of pharmacological sciences, 114(1), 85-91 (2010-08-26)
We examined the in vivo antiviral activities of 2-amino-4,4α-dihydro-4α-7-dimethyl-3H-phenoxazine-3-one (Phx-1), 3-amino-1,4α-dihydro-4α-8-dimethyl-2H-phenoxazine-2-one (Phx-2), and 2-aminophenoxazine-3-one (Phx-3) against herpes viruses. The virus yield three days after administration, changes in the 6-degree's lesion scores, and the morbidity were assessed after herpes simplex virus
Martin Link et al.
Bioorganic & medicinal chemistry letters, 21(18), 5538-5542 (2011-08-02)
Novel amino-reactive phenoxazines were obtained by reasonably simple synthetic protocols and characterized in terms of their use as fluorescent labels for amines, amino acids and proteins in general. Purple labels (alternatives to Texas Red) and blue labels (alternatives to Cy-1)
Karina Mondragón-Vásquez et al.
Chemical communications (Cambridge, England), (44)(44), 6726-6728 (2009-11-04)
N(6)-(N'-Arylcarbamoyl)-2'-deoxyadenosine-H-phosphonates displayed molecular recognition towards cationic phenothiazinium and phenoxazinium dyes in aqueous solutions; studies have shown that binding is driven mainly by aromatic interactions and that size and shape-complementarity of the aromatic rings in host and guest provides selectivity.
Praew Thansandote et al.
The Journal of organic chemistry, 75(10), 3495-3498 (2010-04-29)
A rapid, four-step approach to alkyl- and aryl-substituted benzomorpholines is accomplished by a Pd-catalyzed domino C-C/C-N bond coupling using a norbornene template. Extension to phenoxazines and dihydrodibenzoxazepines is presented.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![10,11-Dihydro-5H-dibenz[b,f]azepine 97%](/deepweb/assets/sigmaaldrich/product/structures/282/468/27ed6f23-3d01-4628-8293-f0051a6f3b7c/640/27ed6f23-3d01-4628-8293-f0051a6f3b7c.png)
