554324
Phenylacetyl disulfide
96%
Synonym(s):
Bis(phenylacetyl) disulfide, Di(phenylacetyl) disulfide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
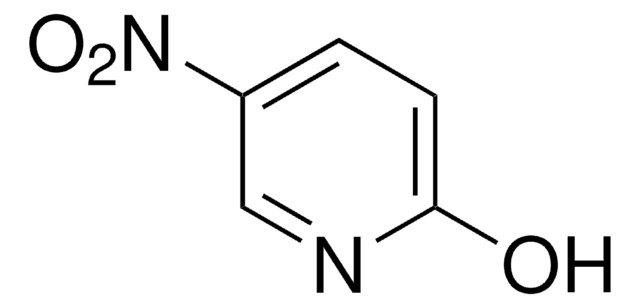
(C6H5CH2COS)2
CAS Number:
Molecular Weight:
302.41
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
solid
mp
59-63 °C (lit.)
SMILES string
O=C(Cc1ccccc1)SSC(=O)Cc2ccccc2
InChI
1S/C16H14O2S2/c17-15(11-13-7-3-1-4-8-13)19-20-16(18)12-14-9-5-2-6-10-14/h1-10H,11-12H2
InChI key
IXGZXXBJSZISOO-UHFFFAOYSA-N
General description
Phenylacetyl disulphide serves as a sulfurization reagent during the preparation of phosphates.Phenyl acetyl disulfide can be synthesized by the reaction of phenyl benzenethiolsulfonate with thioacetic acid in the presence of triethylamine.
Application
Phenylacetyl disulfide (PADS) may be used as a sulphur transfer agent during the synthesis of phosphorothioate oligodeoxyribonucleotides.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A study on the use of phenylacetyl disulfide in the solid-phase synthesis of oligodeoxynucleoside phosphorothioates.
Roelen HCPF, et al.
J. R. Neth. Chem. Soc., 110(7-8), 325-331 (1991)
"Organic disulfides and related substances. XXIII. Unsymmetrical carbonyl disulfides and cognate compounds"
Fiel L and Buckman DJ
The Journal of Organic Chemistry, 32(11), 3467-3470 (1967)
Caged O-phosphorothioyl amino acids as building blocks for Fmoc-based solid phase peptide synthesis.
Andreas Aemissegger et al.
Tetrahedron, 63(27), 6185-6190 (2007-07-02)
The synthesis of 1-(2-nitrophenylethyl) caged O-phosphorothioylserine, -threonine and -tyrosine derivatives is reported. These amino acid building blocks can be directly incorporated into peptides by Fmoc-based solid phase synthesis as their pentafluorophenyl esters or as symmetric anhydrides. Upon irradiation with UV
Use of phenylacetyl disulfide (PADS) in the synthesis of oligodeoxyribonucleotide phosphorothioates.
Cheruvallath ZS, et al.
Nucleosides, nucleotides & nucleic acids, 18(3), 485-492 (1999)
Synthesis of antisense oligonucleotides: Replacement of 3H-1, 2-benzodithiol-3-one 1, 1-dioxide (Beaucage reagent) with phenylacetyl disulfide (PADS) as efficient sulfurization reagent: From bench to bulk manufacture of active pharmaceutical ingredient.
Cheruvallath ZS, et al.
Organic Process Research & Development, 4(3), 199-204 (2000)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service