All Photos(1)
About This Item
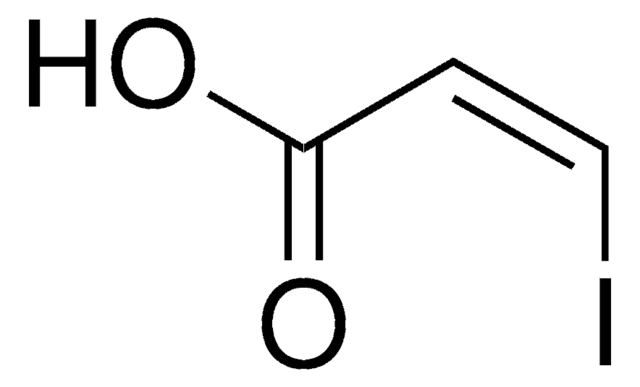
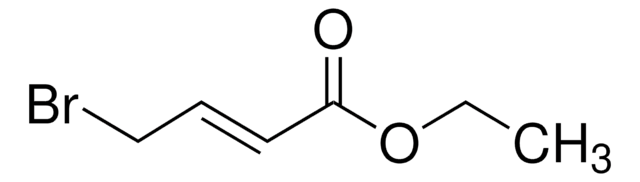
Linear Formula:
ICH=CHCO2C2H5
CAS Number:
Molecular Weight:
226.01
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.533 (lit.)
bp
85 °C/20 mmHg (lit.)
density
1.765 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]\C(I)=C(/[H])C(=O)OCC
InChI
1S/C5H7IO2/c1-2-8-5(7)3-4-6/h3-4H,2H2,1H3/b4-3-
InChI key
AELYFQSZXFFNGP-ARJAWSKDSA-N
General description
Ethyl cis-3-iodoacrylate (cis-ethyl-β-iodoacrylate, ethyl (Z)-β-iodoacrylate) is Z-vinyl iodide derivative. It is reported to be prepared from ethyl propiolate by treating with sodium iodide. Its Suzuki coupling reaction with cyclic vinyl boronic acids has been studied.
Application
Ethyl cis-3-iodoacrylate may be used in the synthesis of the following:
- vinyl sulfides
- (Z)-1-iodohept-1-en-3-ol
- (E)-1-iodohept-1-en-3-ol
- (Z)-β-iodoacrolein
- 6-oxo-M1Guo
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
210.2 °F - closed cup
Flash Point(C)
99 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Stereo-and regiospecific Cu-catalyzed cross-coupling reaction of vinyl iodides and thiols: a very mild and general route for the synthesis of vinyl sulfides.
Kabir MS, et al.
Organic Letters, 10(15), 3363-3366 (2008)
A simple and convenient method for the preparation of (Z)-β-iodoacrolein and of (Z)- or (E)-γ-iodo allylic alcohols: (Z)- and (E)-1-iodohept-1-en-3-ol.
Marek I, et al.
Organic Syntheses, 74, 194-194 (1997)
Selection of monoclonal antibodies against 6-oxo-M1dG and their use in an LC-MS/MS assay for the presence of 6-oxo-M1dG in vivo.
Akingbade D, et al.
Chemical Research in Toxicology, 25(2), 454-461 (2012)
Regiochemical control in the metal-catalyzed transposition of allylic silyl ethers.
Hansen EC and Lee D.
Journal of the American Chemical Society, 128(25), 8142-8143 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service