36295
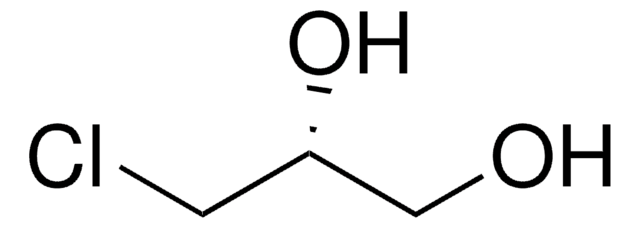
2,3-Dichloro-1-propanol
≥97.0% (GC)
Synonym(s):
Glycerol-α,β-dichlorohydrin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClCH2CHClCH2OH
CAS Number:
Molecular Weight:
128.99
Beilstein:
1732060
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97.0% (GC)
density
1.360 g/mL at 20 °C (lit.)
SMILES string
OCC(Cl)CCl
InChI
1S/C3H6Cl2O/c4-1-3(5)2-6/h3,6H,1-2H2
InChI key
ZXCYIJGIGSDJQQ-UHFFFAOYSA-N
General description
2,3-Dichloro-1-propanol belongs to the group of chloropropanols. Inhibitory effects of 2,3-dichloro-1-propanol on T cell both in vivo and in vitro is reported. Improved enantioselective resolution of (R,S)-2,3-dichloro-1-propanol to (S)-2, 3-dichloro-1-propanol by whole cells of a recombinant Escherichia coli in n-heptane-aqueous biphasic system is reported.
Application
2,3-Dichloro-1-propanol may be employed as carbon and energy supplement for the growth of Pseudomonas putida strain (MC4).
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 2 Dermal - Acute Tox. 3 Oral - Eye Irrit. 2
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point(F)
199.4 °F
Flash Point(C)
93 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Jing Lu et al.
International immunopharmacology, 17(2), 321-328 (2013-07-16)
2,3-Dichloro-1-propanol (2,3-DCP) is a member of a group of chemicals known as chloropropanols. Currently, immunotoxicity of 2,3-DCP has not been reported. In the present study, we studied its inhibitory effects on T cell both in vivo and in vitro. The
K Fujishiro et al.
Fukuoka igaku zasshi = Hukuoka acta medica, 85(8), 247-250 (1994-08-01)
The toxicity of dichloropropanols (DCPs) was investigated by hematological and blood chemical examination. Solutions of two isomers of DCPs, 1,3-dichloro-2-propanol (DC2P) and 2,3-dichloro-1-propanol (DC1P) were dissolved in saline at a concentration of 100 mg/ml and 0.1 ml of each was
Muhammad Irfan Arif et al.
Applied and environmental microbiology, 78(17), 6128-6136 (2012-07-04)
A Pseudomonas putida strain (MC4) that can utilize 2,3-dichloro-1-propanol (DCP) and several aliphatic haloacids and haloalcohols as sole carbon and energy source for growth was isolated from contaminated soil. Degradation of DCP was found to start with oxidation and concomitant
A J Effendi et al.
Applied and environmental microbiology, 66(7), 2882-2887 (2000-07-06)
2,3-Dichloro-1-propanol is more chemically stable than its isomer, 1, 3-dichloro-2-propanol, and is therefore more difficult to degrade. The isolation of bacteria capable of complete mineralization of 2, 3-dichloro-1-propanol was successful only from enrichments at high pH. The bacteria thus isolated
Shu-Ping Zou et al.
Journal of biotechnology, 188, 42-47 (2014-08-26)
The enantioselective resolution of (R,S)-2,3-dichloro-1-propanol ((R,S)-DCP) to (S)-DCP by whole cells of a recombinant Escherichia coli expressing halohydrin dehalogenase (HHDH) activity was limited by product inhibition. To solve this problem to improve the productivity of (S)-DCP, an n-heptane-aqueous biphasic system
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service