225185
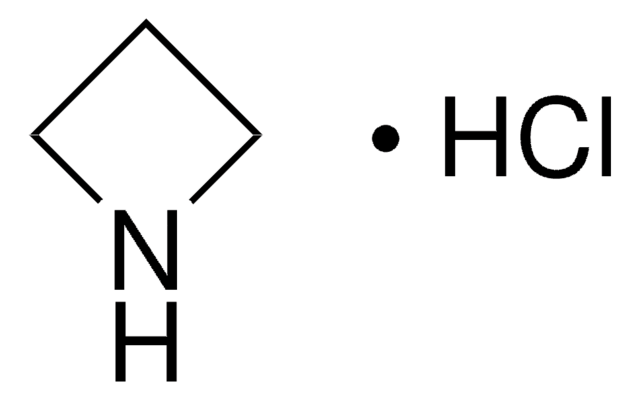
Cyclobutylamine
98%
Synonym(s):
Aminocyclobutane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C4H7NH2
CAS Number:
Molecular Weight:
71.12
Beilstein:
2069297
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
form
liquid
refractive index
n20/D 1.437 (lit.)
bp
81.5 °C/752 mmHg (lit.)
density
0.833 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
NC1CCC1
InChI
1S/C4H9N/c5-4-2-1-3-4/h4H,1-3,5H2
InChI key
KZZKOVLJUKWSKX-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
24.8 °F - closed cup
Flash Point(C)
-4 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
T Maruyama et al.
Chemical & pharmaceutical bulletin, 38(10), 2719-2725 (1990-10-01)
9-Cyclobutyladenine (4a), cis- and trans-9-[3- (hydroxymethyl)cyclobutyl]adenine (4b) and 9-[3,3-bis(hydroxymethyl)cyclobutyl]adenine(4d) were prepared from the corresponding cyclobutylamine derivatives (1a, 1b and 1d). Guanine congeners (9a, cis- and trans-9b and 9d) and carbocyclic oxetanocin G (1',2'-trans-9f) were also prepared. Carbocyclic oxetanocin A(1',2'-trans-4f), the
McNaughton et al.
Journal of molecular spectroscopy, 196(2), 274-282 (1999-11-30)
The infrared spectrum of vinylamine, generated by pyrolysis of cyclobutylamine, has been investigated at low and high resolution. The rovibrational structure of the far infrared spectrum (0.002 cm(-1)) has been analyzed, and effective rotational and centrifugal distortion constants have been
Hartmut Schirok
The Journal of organic chemistry, 71(15), 5538-5545 (2006-07-15)
7-Azaindoles are versatile building blocks, especially in medicinal chemistry, where they serve as bioisosteres of indoles or purines. Herein, we are presenting a robust and flexible synthesis of 1,3- and 1,3,6-substituted 7-azaindoles starting from nicotinic acid derivatives or 2,6-dichloropyridine, respectively.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service

![1-Bicyclo[1.1.1]pentylamine hydrochloride](/deepweb/assets/sigmaaldrich/product/structures/287/052/55f4f60a-a9e0-4ea2-b1e8-5b3f6ce0ff21/640/55f4f60a-a9e0-4ea2-b1e8-5b3f6ce0ff21.png)