This product is tested for solubility in methanol at 50 mg/mL and is also soluble in ethanol and DMSO. The stability in solution has not been determined. However, an identical product, 524488, notes that solutions are stable when stored in aliquots at -20°C for up to 1 month.
P7912
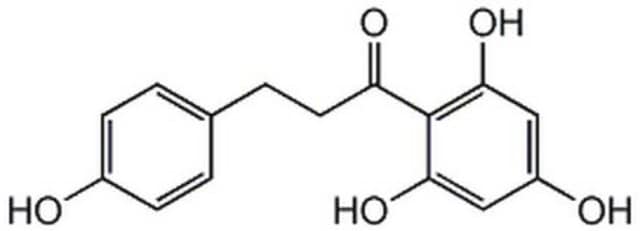
Phloretin
≥99% (HPLC), powder, GLUT inhibitor
Synonyme(s) :
β-(4-Hydroxyphenyl)-2,4,6-trihydroxypropiophenone, 2′,4′,6′-Trihydroxy-3-(4-hydroxyphenyl)propiophenone, 3-(4-Hydroxyphenyl)-1-(2,4,6-trihydroxyphenyl)-1-propanone
About This Item
Produits recommandés
Nom du produit
Phloretin, ≥99%
Niveau de qualité
Essai
≥99%
Forme
powder
Pf
~260 °C
Température de stockage
2-8°C
Chaîne SMILES
Oc1ccc(CCC(=O)c2c(O)cc(O)cc2O)cc1
InChI
1S/C15H14O5/c16-10-4-1-9(2-5-10)3-6-12(18)15-13(19)7-11(17)8-14(15)20/h1-2,4-5,7-8,16-17,19-20H,3,6H2
Clé InChI
VGEREEWJJVICBM-UHFFFAOYSA-N
Vous recherchez des produits similaires ? Visite Guide de comparaison des produits
Description générale
Application
- to study its effect on the 2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-d-glucose (2-NBDG) and 2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)amino]-2-deoxy-l-glucose (2-NBDLG) uptake[2]
- to incubate microvesicles in order to inhibit GLUT1 (glucose transporter 1)-mediated transport in radioactive ligand up-take assay[3]
- as a component of KRH buffer to stop glucose uptake by trophoblast cells in vitro[4]
Actions biochimiques/physiologiques
Mention d'avertissement
Warning
Mentions de danger
Conseils de prudence
Classification des risques
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Organes cibles
Respiratory system
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 2
Point d'éclair (°F)
Not applicable
Point d'éclair (°C)
Not applicable
Équipement de protection individuelle
Eyeshields, Gloves, type N95 (US)
Faites votre choix parmi les versions les plus récentes :
Certificats d'analyse (COA)
Vous ne trouvez pas la bonne version ?
Si vous avez besoin d'une version particulière, vous pouvez rechercher un certificat spécifique par le numéro de lot.
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Les clients ont également consulté
Articles
Warburg effect enhances glucose to lactate conversion in tumor cells, regardless of oxygen levels; impacting cancer metabolism since 1924.
Warburg effect enhances glucose to lactate conversion in tumor cells, regardless of oxygen levels; impacting cancer metabolism since 1924.
Warburg effect enhances glucose to lactate conversion in tumor cells, regardless of oxygen levels; impacting cancer metabolism since 1924.
Warburg effect enhances glucose to lactate conversion in tumor cells, regardless of oxygen levels; impacting cancer metabolism since 1924.
-
この製品の溶解方法と溶解した後の保存方法と、保存可能期間をどうか教えてください。 Please let me know how to dissolve this product, how to store it, and how long it can be stored after dissolving.
1 answer-
Helpful?
-
-
What is the solubility of Phloretin in DMSO?
1 answer-
Stock solutions of phloretin can be prepared in DMSO at 100 mM
Helpful?
-
Active Filters
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique