860464P
Avanti
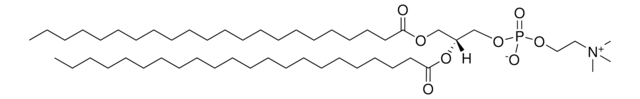
N-C24:1-deoxysphinganine
Avanti Research™ - A Croda Brand 860464P, powder
Synonyme(s) :
N-nervonoyl-1-deoxysphinganine (m18:0/24:1)
About This Item
Produits recommandés
Forme
powder
Conditionnement
pkg of 1 × 1 mg (860464P-1mg)
Fabricant/nom de marque
Avanti Research™ - A Croda Brand 860464P
Type de lipide
sphingolipids
bioactive lipids
Conditions d'expédition
dry ice
Température de stockage
−20°C
Chaîne SMILES
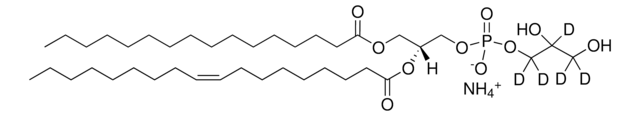
C[C@]([H])(NC(CCCCCCCCCCCCC/C=C\CCCCCCCC)=O)[C@]([H])(O)CCCCCCCCCCCCCCC
Catégories apparentées
Description générale
Application
Actions biochimiques/physiologiques
Conditionnement
Informations légales
Code de la classe de stockage
11 - Combustible Solids
Classe de danger pour l'eau (WGK)
WGK 3
Certificats d'analyse (COA)
Recherchez un Certificats d'analyse (COA) en saisissant le numéro de lot du produit. Les numéros de lot figurent sur l'étiquette du produit après les mots "Lot" ou "Batch".
Déjà en possession de ce produit ?
Retrouvez la documentation relative aux produits que vous avez récemment achetés dans la Bibliothèque de documents.
Notre équipe de scientifiques dispose d'une expérience dans tous les secteurs de la recherche, notamment en sciences de la vie, science des matériaux, synthèse chimique, chromatographie, analyse et dans de nombreux autres domaines..
Contacter notre Service technique


![16:0-d31-18:1 PG 1-palmitoyl-d31-2-oleoyl-sn-glycero-3-[phospho-rac-(1-glycerol)] (sodium salt), chloroform](/deepweb/assets/sigmaaldrich/product/structures/392/823/d104ed37-ed4a-4f66-8294-a20d5f9085b4/640/d104ed37-ed4a-4f66-8294-a20d5f9085b4.png)