89064
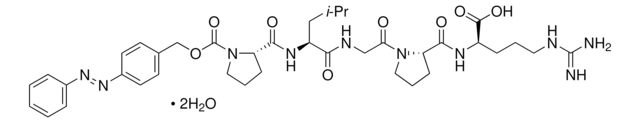
4-Phenylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-D-Arg trifluoroacetate salt
collagenase substrate, chromogenic, ≥95% (HPLC), powder
Synonym(s):
Pz-Pro-Leu-Gly-Pro-D-Arg trifluoroacetate salt
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C38H52N10O8 · xC2HF3O2
Molecular Weight:
776.88 (free base basis)
UNSPSC Code:
12352204
NACRES:
NA.32
Recommended Products
product name
4-Phenylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-D-Arg trifluoroacetate salt, ≥95% (HPLC)
Quality Level
Assay
≥95% (HPLC)
form
powder
Application
4-Phenylazobenzyloxycarbonyl-Pro-Leu-Gly-Pro-D-Arg trifluoroacetate salt has been used as substrate for collagenase.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Study of the Collagen-binding Domain of a 116-kDaClostridium histolyticum Collagenase*
Osamu Matsushita
The Journal of Biological Chemistry (1998)
U Tisljar
Biological chemistry Hoppe-Seyler, 374(2), 91-100 (1993-02-01)
Thimet oligopeptidase (EC 3.4.24.15) is a thiol-dependent metallo-endopeptidase also known as Pz-peptidase, collagenase-like peptidase, endooligopeptidase A, soluble metallo-endopeptidase and endopeptidase 24.15. The enzyme is closely related to the yeast proteinase yscD. Thimet oligopeptidase (M(r) 74000) is widely distributed in animals
T Chikuma et al.
Journal of chromatography, 635(1), 81-87 (1993-04-09)
A rapid and sensitive assay method for the determination of PZ-peptidase activity is reported. This method is based on the monitoring of the absorption at 320 nm of 4-phenylazobenzyloxycarbonyl-L-Pro-L-Leu (PZ-Pro-Leu), enzymatically formed from the substrate 4-phenylazobenzyloxycarbonyl-L-Pro-L-Leu-Gly-L-Pro-D-Arg (PZ-peptide), after separation by
Highly sensitive assay for PZ-peptidase activity by high-performance liquid chromatography
Chikuma, T., et al.
Journal of Chromatography A, 348, 205-212 (1985)
The induction of collagenase and a neutral proteinase by their high molecular weight substrates in Achromobacter iophagus.
V Keil-Dlouha et al.
Journal of molecular biology, 107(3), 293-305 (1976-11-05)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service![N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala](/deepweb/assets/sigmaaldrich/product/structures/805/876/96b5fb57-71c8-4c6b-b5d2-fafe7374cd85/640/96b5fb57-71c8-4c6b-b5d2-fafe7374cd85.png)