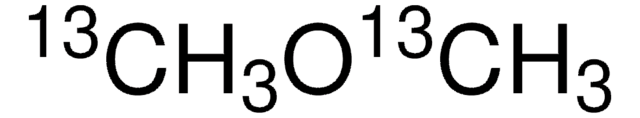38914
Dimethyl ether
puriss., ≥99.9% (GC)
Synonym(s):
Methyl ether
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
(CH3)2O
CAS Number:
Molecular Weight:
46.07
Beilstein:
1730743
EC Number:
MDL number:
UNSPSC Code:
12142100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
vapor density
1.62 (vs air)
vapor pressure
>760 mmHg ( 25 °C)
grade
puriss.
Assay
≥99.9% (GC)
autoignition temp.
662 °F
expl. lim.
27 %
bp
−24.8 °C (lit.)
mp
−141 °C (lit.)
functional group
ether
SMILES string
COC
InChI
1S/C2H6O/c1-3-2/h1-2H3
InChI key
LCGLNKUTAGEVQW-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Packaging
Cylinder with net ~4 kg
DIN 477 nr.1
Other Notes
Sales restrictions may apply
Recommended products
Z742161 DIN 1 Regulator OR 99112 DIN 1 Valve
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Flam. Gas 1A - Press. Gas Liquefied gas
Storage Class Code
2A - Gases
WGK
WGK 1
Flash Point(F)
-41.8 °F - closed cup
Flash Point(C)
-41 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bruce L Yoder et al.
The Journal of chemical physics, 138(4), 044202-044202 (2013-02-08)
We present a new experimental configuration for the study of size-dependent, angle-resolved photoelectron and photoion spectra of weakly bound ultrafine aerosol particles targeted at particle sizes below ~20 nm. It combines single photon ionization by a tunable, table-top vacuum ultraviolet
Hideki Kanda et al.
Water environment research : a research publication of the Water Environment Federation, 83(1), 23-25 (2011-02-05)
We proposed a method for the deodorising and dewatering of biosolids. In the proposed method, liquefied dimethyl ether (DME) was used as an extractant for odorous components and water. We developed a bench-scale experiment to almost completely deodorize and dewater
Xiao Liang et al.
The journal of physical chemistry. B, 115(25), 8199-8206 (2011-06-10)
The acid-catalyzed hydrolysis of dimethyl ether (DME) to methanol was examined using ab initio density functional metadynamics simulations. Diffusion of the acid proton from the aqueous medium, leading to the formation of a protonated DME, is 12.3 kcal mol(-1) activated
Kazuyuki Oshita et al.
Chemosphere, 78(9), 1148-1154 (2010-01-02)
We investigated whether polychlorinated biphenyls (PCBs) and water could be simultaneously removed from river sediment by solvent extraction using liquefied dimethyl ether (DME) as the extractant. DME exists in a gaseous state at normal temperature and pressure and can dissolve
Salma Parveen et al.
The journal of physical chemistry. A, 113(21), 6182-6191 (2009-05-09)
Theoretical calculations have been carried out using ab initio MP2 and B3LYP density functional methods to investigate the interaction between fluorinated dimethyl ethers (nF = 1-5) and water. Depending on the number of F atoms implanted on the dimethyl ethers
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service