31640
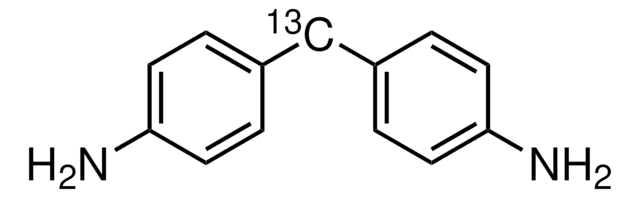
4,4′-Diaminodiphenylmethane
analytical standard
Synonym(s):
DAPM, 4,4′-Methylenedianiline, MDA
About This Item
Recommended Products
grade
analytical standard
Quality Level
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
mp
88-92 °C
application(s)
cleaning products
cosmetics
food and beverages
personal care
format
neat
SMILES string
Nc1ccc(Cc2ccc(N)cc2)cc1
InChI
1S/C13H14N2/c14-12-5-1-10(2-6-12)9-11-3-7-13(15)8-4-11/h1-8H,9,14-15H2
InChI key
YBRVSVVVWCFQMG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
1 of 4
This Item | EHU133791 | EHU111551 | EHU143981 |
|---|---|---|---|
| product line MISSION® | product line MISSION® | product line MISSION® | product line MISSION® |
| esiRNA cDNA target sequence GGACGTGGCCTATCACTGTTACCCCAACAAGTGTAACGACGATGCCCTGGCCTCTAAGGGAGCTCTGTTTCTCAAGGTGCAGGTGGGAAGCACACCTCAGGACCAAAGAATCGTCGGGTACATCGCCTGCACACACCTGCATGCCCCGCAAGAGGACAGCGCCATCCGGTGTGGGCAGCTGGACCTGCTTCAGGACTGGCTGGCTGATTTCCGAAAATCTACCTCCTCGTCCAGCGCAGCCAACCCCGAGGAGCTGGTGGCATTTGACGTCGTCTGTGGAGATTTCAACTTTGATAACTGCTCCTCTGACGACAAGCTGGAGCAGCAACACTCCCTGTTCACCCACTACAGGGACCCCTGCCGCCTGGGGCCTGGTGAGGAGAAGCCGTGGGCCATCGGTACTCTGCTGGACACGAACG | esiRNA cDNA target sequence GCTGTGAGGAGGAGTTCCAGCAACAGAAGTTGGACCTGCTTTGGCAGAAGTTGGTTGACAAGGCTCCACTCAGACAGAAGCACCTCATCTGTGGCCCTGTGAAAGTAGCTGGTGCACCTGGCACTCTGATGACTGCCCCTGAGTACTACGAGTTCCGGCATACCCAGGTGTGCAAGGCCTCAGCACTGAAGCATGGTGGGGATCTGGCACAAGACCCAGCCTGGACAGAGATCTTTGGTGTTCTCTCTGTGGCCACCATCAAGTTTGAGATGCTGAGCACAGCCCCACAGAGTCAGCTCTTCCTGGCTCTGGCTGACAGCAGTATCTCCACGAAGGGCACAAAGAGTGGCACCTTTGTCATGTATAATTGTGCCCGTCTTGCCACACTCTTTGAGAGTTACAAGTGTAGTATGGAACAAGGTCTGTACCCCACTTTTCCTCCTGTGAGC | esiRNA cDNA target sequence GCATCCCCACAGCTAATTTTCCTGAGCAAGTGGTAGATAATCTTCCAGCTGATATATCCACTGGTATTTACTATGGTTGGGCCAGTGTTGGAAGTGGAGATGTCCATAAGATGGTGGTGAGCATAGGATGGAACCCATATTACAAGAATACGAAGAAGTCTATGGAAACACATATCATGCATACCTTCAAAGAGGACTTCTATGGGGAAATCCTCAATGTGGCCATTGTTGGCTACCTGAGACCAGAAAAGAACTTTGATTCTTTAGAGTCACTTATTTCAGCAATTCAAGGTGATATTGAAGAAGCTAAGAAACGACTAGAGTTACCAGAACATTTGAAAATCAAAGAAGACAATTTCTTCCAGGTTTCTAAAAGCAAAATAATGAATGGCCACTGATGA | esiRNA cDNA target sequence TTCTGCTCCTTCTGCCCTAAGAGTTTATGGCCAGTACTTGAATCTTGATAAAGATCACAATGGCATGCTCAGTAAAGAAGAACTCTCACGCTATGGAACAGCTACCATGACCAATGTCTTCTTAGACCGTGTTTTCCAGGAGTGTCTCACTTATGATGGAGAAATGGACTATAAGACCTACTTGGACTTTGTCCTTGCATTAGAAAACAGAAAGGAACCTGCAGCTCTACAATATATTTTCAAACTGCTTGATATTGAGAACAAAGGATACCTGAATGTCTTTTCACTTAATTATTTCTTTAGGGCCATACAGGAACTAATGAAAATCCATGGACAAGATCCTGTTTCATTTCAAGATGTCAAGGATGAAATCTTTGACATGGTAAAACCAAAGGATCCTTTGAAAATCTCTCTTCAGGATTTAATCAACAGTAATCAAGGAGACACAGTAACCACCATTCTAATCGATTTGAATGGCTTCTGGACTTACG |
| Gene Information human ... SMPD3(55512), SMPD3(55512) | Gene Information human ... DALRD3(55152), DALRD3(55152) | Gene Information human ... RFK(55312), RFK(55312) | Gene Information human ... PPP2R3C(55012), C14orf10(55012) |
| storage temp. −20°C | storage temp. −20°C | storage temp. −20°C | storage temp. −20°C |
| form lyophilized powder | form lyophilized powder | form lyophilized powder | form lyophilized powder |
General description
Application
- Urine samples using gas-chromatography coupled to mass spectrometry (GC-MS).[4]
- Hepatic microsomal incubation mixture from rabbits using high-performance liquid chromatography-plasma spray mass spectrometry (HPLC-PSP-MS), offline HPLC with fast atom bombardment mass spectrometry (FAB-MS) and fast atom bombardment tandem mass spectrometry (FAB-MS/MS).[2]
It is reported to be used in the synthesis of amino modified multi-walled carbon nanotubes/polydimethylsiloxane, which serves as a coating for the stir bar sorptive extraction (SBSE) of phenols in environmental samples, followed by analysis using high performance liquid chromatography with ultraviolet detection (HPLC-UV).[5]
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Carc. 1B - Muta. 2 - Skin Sens. 1 - STOT RE 2 - STOT SE 1
Target Organs
Liver, Liver,eye - retina
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
429.8 °F - closed cup
Flash Point(C)
221 °C - closed cup
Personal Protective Equipment
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service