MABE1120
Anti- HSPA8/Hsc70 Antibody, clone N69
clone N69, from mouse
Synonym(s):
HSPA8, Heat shock cognate 71 kDa protein, Heat shock 70 kDa protein 8, HSC70, HSC71, HSC73
About This Item
Recommended Products
biological source
mouse
Quality Level
antibody form
purified immunoglobulin
antibody product type
primary antibodies
clone
N69, monoclonal
species reactivity
mouse, rat, bovine, human
technique(s)
flow cytometry: suitable
immunocytochemistry: suitable
immunohistochemistry: suitable
western blot: suitable
isotype
IgG1κ
NCBI accession no.
UniProt accession no.
shipped in
wet ice
target post-translational modification
unmodified
Gene Information
human ... HSPA8(3312)
Related Categories
General description
Specificity
Immunogen
Application
Epigenetics & Nuclear Function
Nuclear Receptors
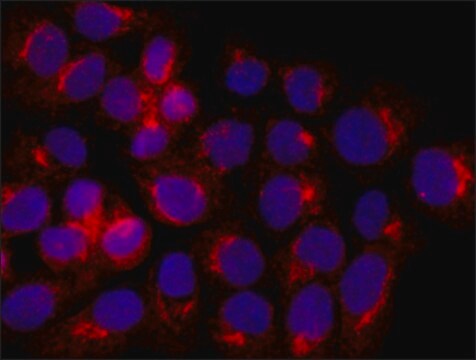
Immunocytochemistry Analysis: 2.0 µg/mL of a representative lot detected the constitutive HSPA8/Hsc70 expression in C6 rat glioma, B16 mouse melanoma, and K-562 human erythroblastoma cells (Courtesy of Dr. Irina V. Guzhova and Dr. Boris Margulis, Russian Academy of Sciences).
Flow Cytometry Analysis: 2.0 µg/mL of a representative lot detected the constitutive HSPA8/Hsc70 expression in C6 rat glioma, B16 mouse melanoma, and K-562 human erythroblastoma cells (Courtesy of Dr. Irina V. Guzhova and Dr. Boris Margulis, Russian Academy of Sciences).
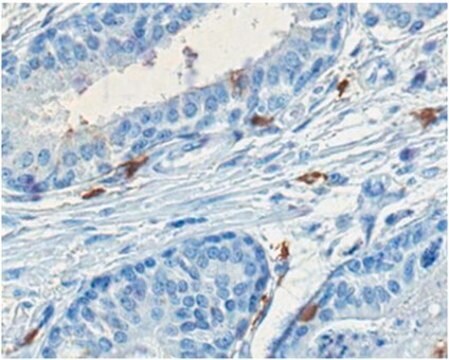
Immunohistochemistry Analysis: A representative lot detected bovine HSPA8/Hsc70 in anterior hypothalamus frozen sections from rats received purified bovine Hsc70 and Hsp72 proteins via intracerebroventricular (i.c.v.) microinjections (Ekimova, I.V., et al. (2010). J. Neurochem. 115(4):1035-1044).
Western Blotting Analysis: A representative lot detected HSPA8/Hsc70 expression in MCF7 cells (Hoyer-Hansen, M., et al. (2005). Cell Death Differ. 12(10):1297-1309).
Western Blotting Analysis: A representative lot detected HSPA8/Hsc70 expression in human breast cancer cell lines (MDA-MB-468, MCF-7, BT-549, and SK-BR-3), non-tumorigenic breast-derived HBL-100 epithelial cells, and WI-38 normal lung fibroblasts (Nylandsted, J., et al. (2000). Proc. Natl. Acad. Sci. U. S. A. 97(14):7871-7876).
Western Blotting Analysis: A representative lot detected HSPA8/Hsc70 (HSC73) purified from bovine skeletal muscle and human myocardium, but not the co-purified HSP72 (Demidov, O.N., et al. (1999). Eur. J. Cardiothorac. Surg. 16(4):444-449).
Quality
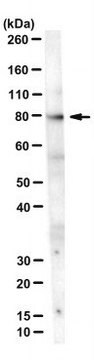
Western Blotting Analysis: 2.0 µg/mL of this antibody detected HSPA8/Hsc70 in 10 µg MCF7 cell lysate.
Target description
Physical form
Storage and Stability
Other Notes
Disclaimer
Not finding the right product?
Try our Product Selector Tool.
Storage Class Code
12 - Non Combustible Liquids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service