All Photos(1)
About This Item
Empirical Formula (Hill Notation):
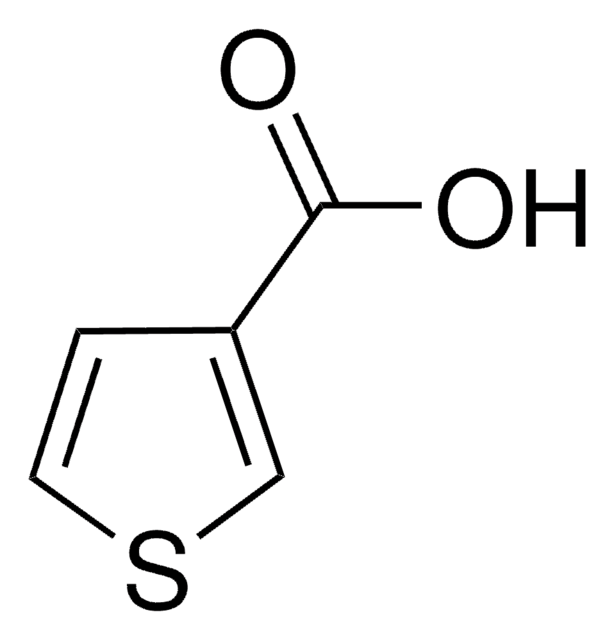
C5H4O2S
CAS Number:
Molecular Weight:
128.15
Beilstein:
110150
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
product line
ReagentPlus®
Assay
99%
bp
260 °C (lit.)
mp
125-127 °C (lit.)
SMILES string
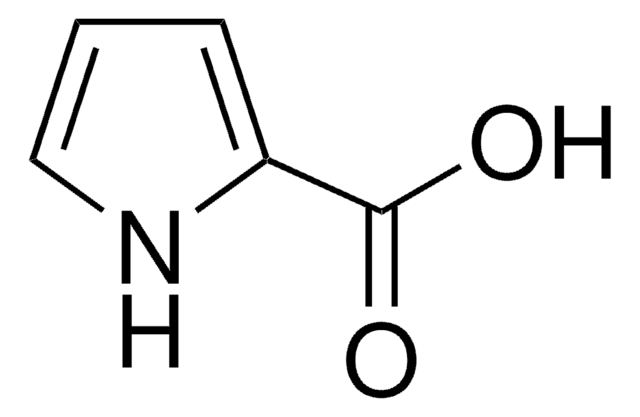
OC(=O)c1cccs1
InChI
1S/C5H4O2S/c6-5(7)4-2-1-3-8-4/h1-3H,(H,6,7)
InChI key
QERYCTSHXKAMIS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Legal Information
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
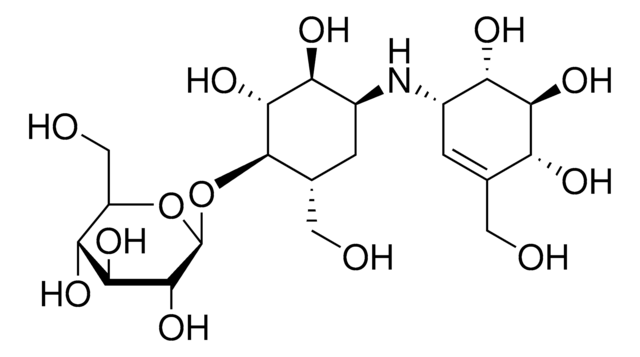
Hao Li et al.
Organic letters, 13(14), 3682-3685 (2011-06-17)
A stereocontrolled synthesis of α-amino-α'-alkoxy ketones is described. This pH-neutral copper(I) thiophene-2-carboxylate (CuTC)-catalyzed cross-coupling of amino acid thiol esters and chiral nonracemic α-alkoxyalkylstannanes gives α-amino-α'-alkoxy ketones in good to excellent yields with complete retention of configuration at the α-amino- and
C Savarin et al.
Organic letters, 3(14), 2149-2152 (2001-07-07)
[reaction: see text] A new protocol for the palladium-catalyzed, copper-mediated coupling of aryl and alkenyl iodides with boronic acids is described. As an alternative to the well-known and widely used Suzuki cross-coupling, this reaction occurs in the absence of a
Laval Chan et al.
Bioorganic & medicinal chemistry letters, 14(3), 797-800 (2004-01-27)
Further SAR studies on the thiophene-2-carboxylic acids are reported. These studies led to the identification of a series of tertiary amides that show inhibition of both HCV NS5B polymerase in vitro and HCV subgenomic RNA replication in Huh-7 cells. Structural
Kerim Babaoglu et al.
Nature chemical biology, 2(12), 720-723 (2006-10-31)
Fragment-based screens test multiple low-molecular weight molecules for binding to a target. Fragments often bind with low affinities but typically have better ligand efficiencies (DeltaG(bind)/heavy atom count) than traditional screening hits. This efficiency, combined with accompanying atomic-resolution structures, has made
C Savarin et al.
Organic letters, 3(1), 91-93 (2001-06-30)
[figure: see text] A new methodology for the synthesis of substituted alkynes is described. In the presence of copper(I) thiophene-2-carboxylate (CuTC) or copper (I) 3-methylsalicylate (CuMeSal), the palladium-catalyzed cross-coupling of thioalkyne derivatives with boronic acids affords functionalized alkynes in yields
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)